PRR5 regulates phosphorylation, nuclear import and subnuclear localization of TOC1 in the Arabidopsis circadian clock
- PMID: 20407420
- PMCID: PMC2885927
- DOI: 10.1038/emboj.2010.76
PRR5 regulates phosphorylation, nuclear import and subnuclear localization of TOC1 in the Arabidopsis circadian clock
Erratum in
- EMBO J. 2010 Sep 1;29(17):3033
Abstract
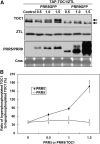
Many core oscillator components of the circadian clock are nuclear localized but how the phase and rate of their entry contribute to clock function is unknown. TOC1/PRR1, a pseudoresponse regulator (PRR) protein, is a central element in one of the feedback loops of the Arabidopsis clock, but how it functions is unknown. Both TOC1 and a closely related protein, PRR5, are nuclear localized, expressed in the same phase, and shorten period when deficient, but their molecular relationship is unclear. Here, we find that both proteins interact in vitro and in vivo through their conserved N-termini. TOC1-PRR5 oligomerization enhances TOC1 nuclear accumulation two-fold, most likely through enhanced nuclear import. In addition, PRR5 recruits TOC1 to large subnuclear foci and promotes phosphorylation of the TOC1 N-terminus. Our results show that nuclear TOC1 is essential for normal clock function and reveal a mechanism to enhance phase-specific TOC1 nuclear accumulation. Interestingly, this process of regulated nuclear import is reminiscent of similar oligomeric pairings in animal clock systems (e.g. timeless/period and clock/cycle), suggesting evolutionary convergence of a conserved mechanism across kingdoms.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




Similar articles
-
REVEILLE8 and PSEUDO-REPONSE REGULATOR5 form a negative feedback loop within the Arabidopsis circadian clock.PLoS Genet. 2011 Mar;7(3):e1001350. doi: 10.1371/journal.pgen.1001350. Epub 2011 Mar 31. PLoS Genet. 2011. PMID: 21483796 Free PMC article.
-
Post-translational regulation of the Arabidopsis circadian clock through selective proteolysis and phosphorylation of pseudo-response regulator proteins.J Biol Chem. 2008 Aug 22;283(34):23073-83. doi: 10.1074/jbc.M803471200. Epub 2008 Jun 18. J Biol Chem. 2008. PMID: 18562312
-
Insight into missing genetic links between two evening-expressed pseudo-response regulator genes TOC1 and PRR5 in the circadian clock-controlled circuitry in Arabidopsis thaliana.Plant Cell Physiol. 2008 Feb;49(2):201-13. doi: 10.1093/pcp/pcm178. Epub 2008 Jan 4. Plant Cell Physiol. 2008. PMID: 18178585
-
Cold-induced degradation of core clock proteins implements temperature compensation in the Arabidopsis circadian clock.Sci Adv. 2024 Sep 27;10(39):eadq0187. doi: 10.1126/sciadv.adq0187. Epub 2024 Sep 27. Sci Adv. 2024. PMID: 39331704 Free PMC article.
-
Phosphorylation in the plant circadian system.Trends Plant Sci. 2012 Oct;17(10):575-83. doi: 10.1016/j.tplants.2012.06.008. Epub 2012 Jul 9. Trends Plant Sci. 2012. PMID: 22784827 Review.
Cited by
-
Post-Translational Mechanisms of Plant Circadian Regulation.Genes (Basel). 2021 Feb 24;12(3):325. doi: 10.3390/genes12030325. Genes (Basel). 2021. PMID: 33668215 Free PMC article. Review.
-
HOS15-mediated turnover of PRR7 enhances freezing tolerance.New Phytol. 2024 Nov;244(3):798-810. doi: 10.1111/nph.20062. Epub 2024 Aug 19. New Phytol. 2024. PMID: 39155726
-
REVEILLE8 and PSEUDO-REPONSE REGULATOR5 form a negative feedback loop within the Arabidopsis circadian clock.PLoS Genet. 2011 Mar;7(3):e1001350. doi: 10.1371/journal.pgen.1001350. Epub 2011 Mar 31. PLoS Genet. 2011. PMID: 21483796 Free PMC article.
-
A Compact Model for the Complex Plant Circadian Clock.Front Plant Sci. 2016 Feb 5;7:74. doi: 10.3389/fpls.2016.00074. eCollection 2016. Front Plant Sci. 2016. PMID: 26904049 Free PMC article.
-
The clock gene circuit in Arabidopsis includes a repressilator with additional feedback loops.Mol Syst Biol. 2012 Mar 6;8:574. doi: 10.1038/msb.2012.6. Mol Syst Biol. 2012. PMID: 22395476 Free PMC article.
References
-
- Alabadi D, Oyama T, Yanovsky MJ, Harmon FG, Mas P, Kay SA (2001) Reciprocal regulation between TOC1 and LHY/CCA1 within the Arabidopsis circadian clock. Science 293: 880–883 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

