Inhibitory "noise"
- PMID: 20407587
- PMCID: PMC2854575
- DOI: 10.3389/fncel.2010.00009
Inhibitory "noise"
Abstract
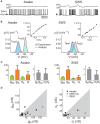
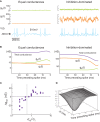
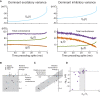
Cortical neurons in vivo may operate in high-conductance states, in which the major part of the neuron's input conductance is due to synaptic activity, sometimes several-fold larger than the resting conductance. We examine here the contribution of inhibition in such high-conductance states. At the level of the absolute conductance values, several studies have shown that cortical neurons in vivo are characterized by strong inhibitory conductances. However, conductances are balanced and spiking activity is mostly determined by fluctuations, but not much is known about excitatory and inhibitory contributions to these fluctuations. Models and dynamic-clamp experiments show that, during high-conductance states, spikes are mainly determined by fluctuations of inhibition, or by inhibitory "noise". This stands in contrast to low-conductance states, in which excitatory conductances determine spiking activity. To determine these contributions from experimental data, maximum likelihood methods can be designed and applied to intracellular recordings in vivo. Such methods indicate that action potentials are indeed mostly correlated with inhibitory fluctuations in awake animals. These results argue for a determinant role for inhibitory fluctuations in evoking spikes, and do not support feed-forward modes of processing, for which opposite patterns are predicted.
Keywords: cerebral cortex; computational models; conductance; dynamic-clamp; spike-triggered average.
Figures
References
-
- Anderson J. S., Carandini M., Ferster D. (2000). Orientation tuning of input conductance, excitation, and inhibition in cat primary visual cortex. J. Neurophysiol. 84, 909–926 - PubMed
-
- Baranyi A., Szente M. B., Woody C. D. (1993). Electrophysiological characterization of different types of neurons recorded in vivo in the motor cortex of the cat. II. Membrane parameters, action potentials, current-induced voltage responses and electrotonic structures. J. Neurophysiol. 69, 1865–1879 - PubMed
-
- Destexhe A. (2007). High-Conductance State. Scholarpedia 2: 1341 Available at: http://www.scholarpedia.org/article/High-Conductance_State
LinkOut - more resources
Full Text Sources

