Heterologous Prime-Boost HIV-1 Vaccination Regimens in Pre-Clinical and Clinical Trials
- PMID: 20407589
- PMCID: PMC2855973
- DOI: 10.3390/v2020435
Heterologous Prime-Boost HIV-1 Vaccination Regimens in Pre-Clinical and Clinical Trials
Abstract
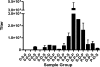
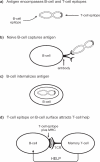
Currently, there are more than 30 million people infected with HIV-1 and thousands more are infected each day. Vaccination is the single most effective mechanism for prevention of viral disease, and after more than 25 years of research, one vaccine has shown somewhat encouraging results in an advanced clinical efficacy trial. A modified intent-to-treat analysis of trial results showed that infection was approximately 30% lower in the vaccine group compared to the placebo group. The vaccine was administered using a heterologous prime-boost regimen in which both target antigens and delivery vehicles were changed during the course of inoculations. Here we examine the complexity of heterologous prime-boost immunizations. We show that the use of different delivery vehicles in prime and boost inoculations can help to avert the inhibitory effects caused by vector-specific immune responses. We also show that the introduction of new antigens into boost inoculations can be advantageous, demonstrating that the effect of `original antigenic sin' is not absolute. Pre-clinical and clinical studies are reviewed, including our own work with a three-vector vaccination regimen using recombinant DNA, virus (Sendai virus or vaccinia virus) and protein. Promising preliminary results suggest that the heterologous prime-boost strategy may possibly provide a foundation for the future prevention of HIV-1 infections in humans.
Figures
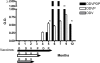
Similar articles
-
Preferential Targeting of Conserved Gag Regions after Vaccination with a Heterologous DNA Prime-Modified Vaccinia Virus Ankara Boost HIV-1 Vaccine Regimen.J Virol. 2017 Aug 24;91(18):e00730-17. doi: 10.1128/JVI.00730-17. Print 2017 Sep 15. J Virol. 2017. PMID: 28701395 Free PMC article.
-
Priming with a Potent HIV-1 DNA Vaccine Frames the Quality of Immune Responses prior to a Poxvirus and Protein Boost.J Virol. 2019 Jan 17;93(3):e01529-18. doi: 10.1128/JVI.01529-18. Print 2019 Feb 1. J Virol. 2019. PMID: 30429343 Free PMC article.
-
Potential To Streamline Heterologous DNA Prime and NYVAC/Protein Boost HIV Vaccine Regimens in Rhesus Macaques by Employing Improved Antigens.J Virol. 2016 Mar 28;90(8):4133-4149. doi: 10.1128/JVI.03135-15. Print 2016 Apr. J Virol. 2016. PMID: 26865719 Free PMC article.
-
Novel prime-boost vaccine strategies against HIV-1.Expert Rev Vaccines. 2019 Aug;18(8):765-779. doi: 10.1080/14760584.2019.1640117. Epub 2019 Jul 9. Expert Rev Vaccines. 2019. PMID: 31271322 Review.
-
Prime-boost vaccine strategy against viral infections: Mechanisms and benefits.Vaccine. 2016 Jan 20;34(4):413-423. doi: 10.1016/j.vaccine.2015.11.062. Epub 2015 Dec 13. Vaccine. 2016. PMID: 26691569 Review.
Cited by
-
Elicitation of both anti HIV-1 Env humoral and cellular immunities by replicating vaccinia prime Sendai virus boost regimen and boosting by CD40Lm.PLoS One. 2012;7(12):e51633. doi: 10.1371/journal.pone.0051633. Epub 2012 Dec 7. PLoS One. 2012. PMID: 23236521 Free PMC article.
-
Optimal priming of poxvirus vector (NYVAC)-based HIV vaccine regimens for T cell responses requires three DNA injections. Results of the randomized multicentre EV03/ANRS VAC20 Phase I/II Trial.PLoS Pathog. 2020 Jun 26;16(6):e1008522. doi: 10.1371/journal.ppat.1008522. eCollection 2020 Jun. PLoS Pathog. 2020. PMID: 32589686 Free PMC article. Clinical Trial.
-
Short Communication: Parallel Analyses of Systemic and Local Vaccinations with Envelope Formulated in Adjuvant for Induction of HIV-Specific Antibodies in the Vaginal Mucosa.AIDS Res Hum Retroviruses. 2017 May;33(5):424-427. doi: 10.1089/AID.2016.0218. Epub 2016 Dec 8. AIDS Res Hum Retroviruses. 2017. PMID: 27825246 Free PMC article.
-
Multi-Envelope HIV-1 Vaccine Development: Two Targeted Immune Pathways, One Desired Protective Outcome.Viral Immunol. 2018 Mar;31(2):124-132. doi: 10.1089/vim.2017.0144. Epub 2018 Jan 9. Viral Immunol. 2018. PMID: 29315059 Free PMC article. Review.
-
Heterologous Prime-Boost Enhances the Antitumor Immune Response Elicited by Plant-Virus-Based Cancer Vaccine.J Am Chem Soc. 2019 Apr 24;141(16):6509-6518. doi: 10.1021/jacs.9b01523. Epub 2019 Apr 16. J Am Chem Soc. 2019. PMID: 30995022 Free PMC article.
References
-
- HIV prevalence estimates--United States, 2006. MMWR Morb Mortal Wkly Rep. 2008;57:1073–1076. - PubMed
-
- McNeil DG., Jr For first time, AIDS Vaccine shows some success. The New York Times. 2009
-
- Cohen J. AIDS vaccines. HIV dodges one-two punch. Science. 2004;305:1545–1547. - PubMed
-
- Burton DR, Desrosiers RC, Doms RW, Feinberg MB, Gallo RC, Hahn B, Hoxie JA, Hunter E, Korber B, Landay A, Lederman MM, Lieberman J, McCune JM, Moore JP, Nathanson N, Picker L, Richman D, Rinaldo C, Stevenson M, Watkins DI, Wolinsky SM, Zack JA. Public health. A sound rationale needed for phase III HIV-1 vaccine trials. Science. 2004;303:316. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
