Dendritic excitability modulates dendritic information processing in a purkinje cell model
- PMID: 20407613
- PMCID: PMC2856590
- DOI: 10.3389/fncom.2010.00006
Dendritic excitability modulates dendritic information processing in a purkinje cell model
Abstract
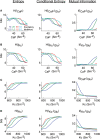
Using an electrophysiological compartmental model of a Purkinje cell we quantified the contribution of individual active dendritic currents to processing of synaptic activity from granule cells. We used mutual information as a measure to quantify the information from the total excitatory input current (I(Glu)) encoded in each dendritic current. In this context, each active current was considered an information channel. Our analyses showed that most of the information was encoded by the calcium (I(CaP)) and calcium activated potassium (I(Kc)) currents. Mutual information between I(Glu) and I(CaP) and I(Kc) was sensitive to different levels of excitatory and inhibitory synaptic activity that, at the same time, resulted in the same firing rate at the soma. Since dendritic excitability could be a mechanism to regulate information processing in neurons we quantified the changes in mutual information between I(Glu) and all Purkinje cell currents as a function of the density of dendritic Ca (g(CaP)) and Kca (g(Kc)) conductances. We extended our analysis to determine the window of temporal integration of I(Glu) by I(CaP) and I(Kc) as a function of channel density and synaptic activity. The window of information integration has a stronger dependence on increasing values of g(Kc) than on g(CaP), but at high levels of synaptic stimulation information integration is reduced to a few milliseconds. Overall, our results show that different dendritic conductances differentially encode synaptic activity and that dendritic excitability and the level of synaptic activity regulate the flow of information in dendrites.
Keywords: cerebellum; compartmental modeling; dendritic computation; dendritic conductances; firing rate; information theory; modulatory synapses; synaptic plasticity.
Figures
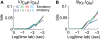
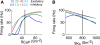

Similar articles
-
Synaptic integration in a model of cerebellar granule cells.J Neurophysiol. 1994 Aug;72(2):999-1009. doi: 10.1152/jn.1994.72.2.999. J Neurophysiol. 1994. PMID: 7527078
-
Dendritic Kv3.3 potassium channels in cerebellar purkinje cells regulate generation and spatial dynamics of dendritic Ca2+ spikes.J Neurophysiol. 2010 Jun;103(6):3516-25. doi: 10.1152/jn.00982.2009. Epub 2010 Mar 31. J Neurophysiol. 2010. PMID: 20357073 Free PMC article.
-
An active membrane model of the cerebellar Purkinje cell II. Simulation of synaptic responses.J Neurophysiol. 1994 Jan;71(1):401-19. doi: 10.1152/jn.1994.71.1.401. J Neurophysiol. 1994. PMID: 8158238
-
The integrative properties of spiny distal dendrites.Neuroscience. 1992;47(3):495-519. doi: 10.1016/0306-4522(92)90161-t. Neuroscience. 1992. PMID: 1584406 Review.
-
Synaptic integration in dendritic trees.J Neurobiol. 2005 Jul;64(1):75-90. doi: 10.1002/neu.20144. J Neurobiol. 2005. PMID: 15884003 Review.
Cited by
-
Neuronal spike timing adaptation described with a fractional leaky integrate-and-fire model.PLoS Comput Biol. 2014 Mar 27;10(3):e1003526. doi: 10.1371/journal.pcbi.1003526. eCollection 2014 Mar. PLoS Comput Biol. 2014. PMID: 24675903 Free PMC article.
-
Single-neuron criticality optimizes analog dendritic computation.Sci Rep. 2013 Nov 14;3:3222. doi: 10.1038/srep03222. Sci Rep. 2013. PMID: 24226045 Free PMC article.
-
The effect of neural noise on spike time precision in a detailed CA3 neuron model.Comput Math Methods Med. 2012;2012:595398. doi: 10.1155/2012/595398. Epub 2012 Jun 24. Comput Math Methods Med. 2012. PMID: 22778784 Free PMC article.
-
The 40-year history of modeling active dendrites in cerebellar Purkinje cells: emergence of the first single cell "community model".Front Comput Neurosci. 2015 Oct 20;9:129. doi: 10.3389/fncom.2015.00129. eCollection 2015. Front Comput Neurosci. 2015. PMID: 26539104 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

