Cholinergic neuromodulation controls directed temporal communication in neocortex in vitro
- PMID: 20407636
- PMCID: PMC2856628
- DOI: 10.3389/fncir.2010.00008
Cholinergic neuromodulation controls directed temporal communication in neocortex in vitro
Abstract
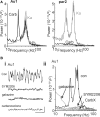
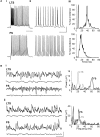
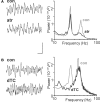
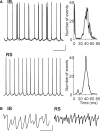
Acetylcholine is the primary neuromodulator involved in cortical arousal in mammals. Cholinergic modulation is involved in conscious awareness, memory formation and attention - processes that involve intercommunication between different cortical regions. Such communication is achieved in part through temporal structuring of neuronal activity by population rhythms, particularly in the beta and gamma frequency ranges (12-80 Hz). Here we demonstrate, using in vitro and in silico models, that spectrally identical patterns of beta2 and gamma rhythms are generated in primary sensory areas and polymodal association areas by fundamentally different local circuit mechanisms: Glutamatergic excitation induced beta2 frequency population rhythms only in layer 5 association cortex whereas cholinergic neuromodulation induced this rhythm only in layer 5 primary sensory cortex. This region-specific sensitivity of local circuits to cholinergic modulation allowed for control of the extent of cortical temporal interactions. Furthermore, the contrasting mechanisms underlying these beta2 rhythms produced a high degree of directionality, favouring an influence of association cortex over primary auditory cortex.
Keywords: EEG; attention; auditory; coherence; interneuron; oscillation.
Figures

Similar articles
-
Potential Mechanisms Underlying Intercortical Signal Regulation via Cholinergic Neuromodulators.J Neurosci. 2015 Nov 11;35(45):15000-14. doi: 10.1523/JNEUROSCI.0629-15.2015. J Neurosci. 2015. PMID: 26558772 Free PMC article.
-
Cholinergic circuit modulation through differential recruitment of neocortical interneuron types during behaviour.J Physiol. 2014 Oct 1;592(19):4155-64. doi: 10.1113/jphysiol.2014.273862. Epub 2014 May 30. J Physiol. 2014. PMID: 24879871 Free PMC article. Review.
-
Dual γ rhythm generators control interlaminar synchrony in auditory cortex.J Neurosci. 2011 Nov 23;31(47):17040-51. doi: 10.1523/JNEUROSCI.2209-11.2011. J Neurosci. 2011. PMID: 22114273 Free PMC article.
-
Synaptic Release of Acetylcholine Rapidly Suppresses Cortical Activity by Recruiting Muscarinic Receptors in Layer 4.J Neurosci. 2018 Jun 6;38(23):5338-5350. doi: 10.1523/JNEUROSCI.0566-18.2018. Epub 2018 May 8. J Neurosci. 2018. PMID: 29739869 Free PMC article.
-
Region-specific changes in gamma and beta2 rhythms in NMDA receptor dysfunction models of schizophrenia.Schizophr Bull. 2008 Sep;34(5):962-73. doi: 10.1093/schbul/sbn059. Epub 2008 Jun 9. Schizophr Bull. 2008. PMID: 18544550 Free PMC article. Review.
Cited by
-
Pharmacological Mechanisms of Cortical Enhancement Induced by the Repetitive Pairing of Visual/Cholinergic Stimulation.PLoS One. 2015 Oct 29;10(10):e0141663. doi: 10.1371/journal.pone.0141663. eCollection 2015. PLoS One. 2015. PMID: 26513575 Free PMC article.
-
A Computational Analysis of the Function of Three Inhibitory Cell Types in Contextual Visual Processing.Front Comput Neurosci. 2017 Apr 25;11:28. doi: 10.3389/fncom.2017.00028. eCollection 2017. Front Comput Neurosci. 2017. PMID: 28487644 Free PMC article.
-
Low-Beta Oscillations Turn Up the Gain During Category Judgments.Cereb Cortex. 2018 Jan 1;28(1):116-130. doi: 10.1093/cercor/bhw356. Cereb Cortex. 2018. PMID: 29253255 Free PMC article.
-
Delta-9-tetrahydrocannabinol, neural oscillations above 20 Hz and induced acute psychosis.Psychopharmacology (Berl). 2015 Feb;232(3):519-28. doi: 10.1007/s00213-014-3684-1. Epub 2014 Jul 20. Psychopharmacology (Berl). 2015. PMID: 25038870 Free PMC article.
-
Rhythms for Cognition: Communication through Coherence.Neuron. 2015 Oct 7;88(1):220-35. doi: 10.1016/j.neuron.2015.09.034. Neuron. 2015. PMID: 26447583 Free PMC article. Review.
References
-
- Cunningham M. O., Whittington M. A., Bibbig A., Roopun A. K., LeBeau F. E. N., Vogt M., Monyer H., Buhl E. H., Traub R. D. (2004). A role for fast rhythmic bursting neurons in cortical gamma oscillations in vitro. Proc. Natl. Acad. Sci. U.S.A. 101, 7152–715710.1073/pnas.0402060101 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

