Clinical trials on CIK cells: first report of the international registry on CIK cells (IRCC)
- PMID: 20407789
- PMCID: PMC11828187
- DOI: 10.1007/s00432-010-0887-7
Clinical trials on CIK cells: first report of the international registry on CIK cells (IRCC)
Abstract
Purpose: Recently, a subset of natural killer T lymphocytes termed "cytokine-induced killer (CIK) cells" has been described. To build an international registry, we collected the clinical data and treatment of patients with cancer using CIK cells from the literature and the respective investigators. This registry is expected to set a new set of standards on the reporting of results from clinical trials using CIK cells. A standardized reporting system will accelerate discoveries and allows us to improve treatment to benefit the patients.
Methods: We searched in PubMed for "CIK cells clinical trials".
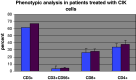
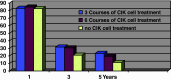
Results: Within the 867 matches found, 11 clinical trials with CIK cells were identified. Within these trials, 426 patients were treated, of which 313 were male, and 113 were female. Most trials included male patients with hepatocellular carcinoma, gastric cancer, and Hodgkin or non-Hodgkin disease. In 10 of 11 studies, autologous CIK cells were used. The total number of CIK cells injected ranged from 21.9 × 10(7) to 5.2 × 10(10). The number of CIK cells used per infusion ranged from 7.2 × 10(6) to 2.1 × 10(10). Patients were treated with up to 40 infusions of CIK cells. Of the 384 patients, where a clinical response was reported, 24 patients showed a complete response, 27 patients showed a partial response, 40 patients showed a minor response. The total response rate (RR) was 91/384 reported patients, 161 patients had a stable disease, 129 patients had progressive disease. A decrease in tumor volume was only described in three patients. Side effects of CIK cell treatment were minor. Interestingly, a reduction of hepatitis B virus load was described in patients undergoing treatment with CIK cells. Disease-free survival rates were significantly higher in patients treated with CIK cells than in a control group without CIK treatment.
Conclusion: Adjuvant immunotherapy with cytokine-induced killer cells may prevent recurrence and improve quality of life and progression-free survival rates in patients with cancer.
Figures
References
-
- Kim HM, Lim J, Park S-K, Kang JS, Lee K, Lee CW et al (2007) Antitumour activity of cytokine-induced killer cells against human lung cancer. http://www.sciencedirect.com: International Immunopharmacology - PubMed
-
- Lu PH, Negrin RS (1994) A novel population of expanded human CD 3+ CD 56+ cells derived from T cells with potent in vivo antitumour activity in SCID mice. J Immunol 153:1687–1696 - PubMed
-
- Schmidt-Wolf IGH, Finke S, Trojaneck B, Denkena A, Lefterova P, Schwella N, Heuft H-G, Prange G, Korte M, Takeya M, Dorbic T, Neubauer A, Wittig B, Huhn D (1999) Phase 1 clinical study applying autologous immunological effector cells transfected with the interleukin-2 gene in patients with metastatic renal cancer, colorectal cancer and lymphoma. Br J Cancer 81(6):1009–1016 - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

