The herbivore-induced plant volatile methyl salicylate negatively affects attraction of the parasitoid Diadegma semiclausum
- PMID: 20407809
- PMCID: PMC2866304
- DOI: 10.1007/s10886-010-9787-1
The herbivore-induced plant volatile methyl salicylate negatively affects attraction of the parasitoid Diadegma semiclausum
Abstract
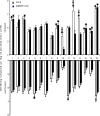
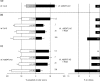
The indirect defense mechanisms of plants comprise the production of herbivore-induced plant volatiles that can attract natural enemies of plant attackers. One of the often emitted compounds after herbivory is methyl salicylate (MeSA). Here, we studied the importance of this caterpillar-induced compound in the attraction of the parasitoid wasp Diadegma semiclausum by using a mutant Arabidopsis line. Pieris rapae infested AtBSMT1-KO mutant Arabidopsis plants, compromised in the biosynthesis of MeSA, were more attractive to parasitoids than infested wild-type plants. This suggests that the presence of MeSA has negative effects on parasitoid host-finding behavior when exposed to wild-type production of herbivore-induced Arabidopsis volatiles. Furthermore, in line with this, we recorded a positive correlation between MeSA dose and repellence of D. semiclausum when supplementing the headspace of caterpillar-infested AtBSMT1-KO plants with synthetic MeSA.
Figures
Similar articles
-
Terpenoid biosynthesis in Arabidopsis attacked by caterpillars and aphids: effects of aphid density on the attraction of a caterpillar parasitoid.Oecologia. 2017 Dec;185(4):699-712. doi: 10.1007/s00442-017-3985-2. Epub 2017 Oct 20. Oecologia. 2017. PMID: 29052769 Free PMC article.
-
Does Aphid Infestation Interfere with Indirect Plant Defense against Lepidopteran Caterpillars in Wild Cabbage?J Chem Ecol. 2017 May;43(5):493-505. doi: 10.1007/s10886-017-0842-z. Epub 2017 Apr 12. J Chem Ecol. 2017. PMID: 28405915 Free PMC article.
-
Multidisciplinary approach to unravelling the relative contribution of different oxylipins in indirect defense of Arabidopsis thaliana.J Chem Ecol. 2009 Sep;35(9):1021-31. doi: 10.1007/s10886-009-9696-3. Epub 2009 Oct 2. J Chem Ecol. 2009. PMID: 19798534 Free PMC article.
-
Attraction of parasitic wasps by caterpillar-damaged plants.Novartis Found Symp. 1999;223:21-32; discussion 32-8. doi: 10.1002/9780470515679.ch3. Novartis Found Symp. 1999. PMID: 10549546 Review.
-
The effects of plant domestication on the foraging and performance of parasitoids.Curr Opin Insect Sci. 2023 Jun;57:101031. doi: 10.1016/j.cois.2023.101031. Epub 2023 Apr 5. Curr Opin Insect Sci. 2023. PMID: 37028646 Review.
Cited by
-
Methyl Salicylate Increases Attraction and Function of Beneficial Arthropods in Cranberries.Insects. 2019 Nov 25;10(12):423. doi: 10.3390/insects10120423. Insects. 2019. PMID: 31775223 Free PMC article.
-
The prospect of applying chemical elicitors and plant strengtheners to enhance the biological control of crop pests.Philos Trans R Soc Lond B Biol Sci. 2014 Feb 17;369(1639):20120283. doi: 10.1098/rstb.2012.0283. Print 2014 Apr 5. Philos Trans R Soc Lond B Biol Sci. 2014. PMID: 24535390 Free PMC article.
-
Variation in herbivory-induced volatiles among cucumber (Cucumis sativus L.) varieties has consequences for the attraction of carnivorous natural enemies.J Chem Ecol. 2011 Feb;37(2):150-60. doi: 10.1007/s10886-011-9906-7. Epub 2011 Jan 20. J Chem Ecol. 2011. PMID: 21249432 Free PMC article.
-
Role of methyl salicylate on oviposition deterrence in Arabidopsis thaliana.J Chem Ecol. 2014 Jul;40(7):754-9. doi: 10.1007/s10886-014-0470-9. Epub 2014 Jun 29. J Chem Ecol. 2014. PMID: 24973956
-
Aphid-Induced Volatiles and Subsequent Attraction of Natural Enemies Varies among Sorghum Cultivars.J Chem Ecol. 2024 Jun;50(5-6):262-275. doi: 10.1007/s10886-024-01493-y. Epub 2024 Apr 22. J Chem Ecol. 2024. PMID: 38647585
References
-
- ADAMS RP. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry. Illinois: Allured Publishing Corporation; 1995.
-
- BENJAMINI Y, HOCHBERG Y. Controlling the false discovery rate—A practical and powerful approach to multiple testing. J. Roy. Stat. Soc. Ser. B. (Stat. Method.) 1995;57:289–300.
-
- BERNASCONI ML, TURLINGS TCJ, AMBROSETTI L, BASSETTI P, DORN S. Herbivore-induced emissions of maize volatiles repel the corn leaf aphid, Rhopalosiphum maidis. Entomol. Exp. Appl. 1998;87:133–142. doi: 10.1023/A:1003200108763. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

