Changes in the levels of some acute-phase proteins in human immunodeficiency virus-1 infected patients, following interleukin-2 treatment
- PMID: 20408859
- PMCID: PMC2940158
- DOI: 10.1111/j.1365-2249.2010.04145.x
Changes in the levels of some acute-phase proteins in human immunodeficiency virus-1 infected patients, following interleukin-2 treatment
Abstract
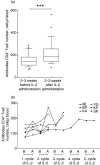
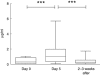
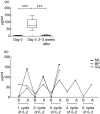
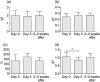
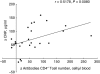
Intermittent interleukin (IL)-2 administration to human immunodeficiency virus (HIV)-1 infected patients is well documented and generally used, but there is limited information about the changes of acute-phase protein (APP) levels in response to this treatment. Fifteen patients undergoing highly active anti-retroviral therapy (HAART) treatment, with undetectable viral load, but low CD4+ cell count (<300/microl), have been treated with 3.6 M IU Proleukine administered twice daily by subcutaneous injection over 5 days. C-reactive protein (CRP), D-dimer, C3, C9, C1-inh and alpha-2HS glycoprotein levels were measured immediately before IL-2 administration, as well as on day 5 and 2-3 weeks thereafter. After IL-2 administration, both mean D-dimer and CRP levels increased significantly (P<0.001), but returned (P<0.001) to baseline within the subsequent 2-3 weeks. Alpha-2HS glycoprotein decreased immediately after IL-2 administration. No significant differences were detected in the levels of C3, C9 and C1-inh. A significant, positive correlation (r=0.5178, P=0.0008) was ascertained between the changes of CRP level, measured immediately before as well as 5 days after IL-2 administration, and changes in CD4 T cell counts measured 2-3 weeks before and after treatment, respectively. IL-2 administration induces rapid elevation of two major APPs (CRP, D-dimer). The positive correlation observed between the changes of CRP levels and CD4+ cell counts after IL-2 administration may indicate that the abrupt, but transitory overproduction of CRP might contribute to the CD4+ cell count-increasing effect of the drug and/ or may be associated with serious side effects.
Figures
References
-
- Smith KA. Interleukin-2: inception, impact, and implications. Science. 1988;240:1169–76. - PubMed
-
- Vogler MA, Teppler H, Gelman R, et al. Daily low-dose subcutaneous interleukin-2 added to single- or dual-nucleoside therapy in HIV infection does not protect against CD4+ T-cell decline or improve other indices of immune function: results of a randomized controlled clinical trial (ACTG 248) J Acquir Immune Defic Syndr. 2004;36:576–87. - PubMed
-
- Emery S, Abrams DI, Cooper DA, et al. The evaluation of subcutaneous proleukin (interleukin-2) in a randomized international trial: rationale, design, and methods of ESPRIT. Control Clin Trials. 2002;23:198–220. - PubMed
-
- Durier C, Capitant C, Lascaux AS, et al. Long-term effects of intermittent interleukin-2 therapy in chronic HIV-infected patients (ANRS 048-079 Trials) Aids. 2007;21:1887–97. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

