Polymerase chain reaction (PCR) detection of B cell clonality in Sjögren's syndrome patients: a diagnostic tool of clonal expansion
- PMID: 20408860
- PMCID: PMC2940149
- DOI: 10.1111/j.1365-2249.2010.04144.x
Polymerase chain reaction (PCR) detection of B cell clonality in Sjögren's syndrome patients: a diagnostic tool of clonal expansion
Abstract
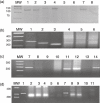
Sjögren's syndrome (SS) is an autoimmune disease characterized by clonal B cell attack of the exocrine glands and dysregulated expression of B cell-activating factor (BAFF). Based upon the current data of increased rates of lymphoid malignancy, as non-Hodgkin's lymphoma (NHL) is associated with SS, we propose the detection of clonal rearrangements of immunoglobulin heavy chain (IgH) gene in those patients as a predictor of malignant clonal expansion. To test our proposal, we examined the IgH clonal rearrangements in SS patients (60) and healthy control subjects (42) having chronic non-specific sialadenitis, to determine the presence of clonal B cells in minor labial salivary glands (MSG) of SS patients. Clonal B cell expansion was assessed by two polymerase chain reaction (PCR) assays: (i) semi-nested PCR, against sequences encoding framework regions FR3, FR2 and FR1c of the variable chain IgH gene in B cells present in the MSG infiltrate; and (ii) the PCR-enzyme-linked immunosorbent assay (ELISA) technique, against the major and minor breakpoint regions of the Bcl-2 oncogene coupled with a variable segment of the IgH to assess the Bcl-2/JH translocation. When FR3, FR2 and FR1c primers were employed, we detected B cell monoclonality in 87% of the SS patients and 19% of the control subjects. The association between inflammation severity of the MSG pattern and the presence of B cell clonality was found to be statistically significant (P<0.01). We concluded that the presence of B cell clonality in MSG can be used as a index of an altered microenvironment favouring the development of lymphoma in SS patients.
Figures

Similar articles
-
Analysis of B-cell clonality in the hepatic tissue of patients with Sjögren's syndrome.Scand J Rheumatol. 2003;32(5):268-72. doi: 10.1080/03009740310003884. Scand J Rheumatol. 2003. PMID: 14690138
-
Molecular B-cell clonality assay in minor salivary glands as a useful tool for the lymphoma risk assessment in Sjögren's syndrome.Joint Bone Spine. 2024 May;91(3):105686. doi: 10.1016/j.jbspin.2023.105686. Epub 2023 Dec 29. Joint Bone Spine. 2024. PMID: 38161050
-
Immunoglobulin gene rearrangements in lymphoplasmacytic infiltrates of labial salivary glands in Sjögren's syndrome. A possible predictor of lymphoma development.Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995 Jun;79(6):723-9. doi: 10.1016/s1079-2104(05)80307-5. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995. PMID: 7621030
-
Clonality analysis of lymphoproliferative disorders in patients with Sjögren's syndrome.Clin Exp Immunol. 2007 Nov;150(2):279-84. doi: 10.1111/j.1365-2249.2007.03486.x. Clin Exp Immunol. 2007. PMID: 17937678 Free PMC article. Review.
-
[Lymphoproliferative disorders in Sjögren's syndrome].Otolaryngol Pol. 2005;59(4):559-64. Otolaryngol Pol. 2005. PMID: 16273862 Review. Polish.
Cited by
-
Predicting the risk for lymphoma development in Sjogren syndrome: An easy tool for clinical use.Medicine (Baltimore). 2016 Jun;95(25):e3766. doi: 10.1097/MD.0000000000003766. Medicine (Baltimore). 2016. PMID: 27336863 Free PMC article.
-
Enzyme-labeled Antigen Method: Development and Application of the Novel Approach for Identifying Plasma Cells Locally Producing Disease-specific Antibodies in Inflammatory Lesions.Acta Histochem Cytochem. 2016 Feb 27;49(1):7-19. doi: 10.1267/ahc.15030. Epub 2016 Feb 26. Acta Histochem Cytochem. 2016. PMID: 27006517 Free PMC article. Review.
-
Persistence and selection of an expanded B-cell clone in the setting of rituximab therapy for Sjögren's syndrome.Arthritis Res Ther. 2014 Feb 11;16(1):R51. doi: 10.1186/ar4481. Arthritis Res Ther. 2014. PMID: 24517398 Free PMC article. Clinical Trial.
-
Possible Mechanisms of Lymphoma Development in Sjögren's Syndrome.Curr Immunol Rev. 2013 Feb;9(1):13-22. doi: 10.2174/1573395511309010003. Curr Immunol Rev. 2013. PMID: 23853604 Free PMC article.
-
Russell body duodenitis with immunoglobulin kappa light chain restriction.World J Gastrointest Endosc. 2015 Jan 16;7(1):73-6. doi: 10.4253/wjge.v7.i1.73. World J Gastrointest Endosc. 2015. PMID: 25610537 Free PMC article.
References
-
- Fox RI, Howell FV, Bone RC, Michelson P. Primary Sjögren syndrome: clinical and immunopathologic features. Semin Arthritis Rheum. 1984;14:77–105. - PubMed
-
- Anaya J-M, Vega P, Correa PA, et al. Síndrome de Sjögren. In: Anaya JM, Shoenfeld Y, Correa PA, García-Carrasco M, Cervera R, editors. Autoinmunidad y Enfermedad Autoinmune [Autoimmunity and Autoimmune Disease] Medellin: Biological Research Corporation; 2005. pp. 295–315.
-
- Martin T, Weber JC, Levallois H, et al. Salivary gland lymphomas in patients with Sjögren's syndrome may frequently develop from rheumatoid factor B cells. Arthritis Rheum. 2000;43:908–16. - PubMed
-
- Szodoray P, Jonsson R. The BAFF/APRIL system in systemic autoimmune diseases with a special emphasis on Sjögren's syndrome. Scand J Immunol. 2005;62:421–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

