Oral non-typable Haemophilus influenzae enhances physiological mechanism of airways protection
- PMID: 20408861
- PMCID: PMC2940157
- DOI: 10.1111/j.1365-2249.2010.04142.x
Oral non-typable Haemophilus influenzae enhances physiological mechanism of airways protection
Abstract
Oral immunotherapy with inactivated non-typeable Haemophilus influenzae (NTHi) prevents exacerbations of chronic obstructive pulmonary disease, but the mechanism is unclear. The aim of this study was to determine the mechanism of protection. This was a placebo versus active prospective study over 3 months in 64 smokers. The active treatment was three courses of oral NTHi given at monthly intervals, followed by measurement of bacteriological and immunological parameters. The results can be summarized: (i) NTHi-specific T cells increased in the placebo treatment group over time (P<0.05); (ii) the T cell response in the oral NTHi group started earlier than that in the placebo group (P<0.05); and (iii) serum NTHi-specific immunoglobulin (Ig)G had significantly greater variation in the placebo group (P<0.0001). The increase in antibody in placebos over time correlated with exposure to live H. influenzae (P<0.05) determined from culture of gargles; (iv) reduction in saliva lysozyme over time (P<0.05) was detected only in the oral NTHi treatment group. These data are consistent with T cell priming of gut lymphoid tissue by aspiration of bronchus content into the gut, with oral immunotherapy augmenting this process leading to enhanced bronchus protection. The evidence for protection was a stable IgG antibody level through the study in the oral NTHi treatment group, contrasting with an increase in antibody correlating with exposure of the airways to H. influenzae in the placebo group. Saliva lysozyme was a useful biomarker of mucosal inflammation, falling after oral NTHi consistent with a reduction in the level of intralumenal inflammation.
Figures

 ) treatments groups is shown. Error bars are standard error of the mean.
) treatments groups is shown. Error bars are standard error of the mean.
 ) treatment groups for subjects with H. influenzae detected in nil or one gargle sample, or those with H. influenzae detected in two to six gargle samples. In the placebo group there were 23 subjects with nil to one and seven subjects with two to six positive gargles. In the HI-164-OV group there were 24 subjects with nil to one and six subjects with two to six positive gargles.
) treatment groups for subjects with H. influenzae detected in nil or one gargle sample, or those with H. influenzae detected in two to six gargle samples. In the placebo group there were 23 subjects with nil to one and seven subjects with two to six positive gargles. In the HI-164-OV group there were 24 subjects with nil to one and six subjects with two to six positive gargles.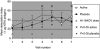
 ) and active (□) treatment groups is shown for each visit. The stimulation index (SI) for the active treatment group is significantly different (P < 0·05) at visits 4, 5 and 7 to that at baseline (visit 1). The SI for the placebo treatment group is significantly different (P < 0·05) at visits 4 and 5 to that at baseline (visit 1).
) and active (□) treatment groups is shown for each visit. The stimulation index (SI) for the active treatment group is significantly different (P < 0·05) at visits 4, 5 and 7 to that at baseline (visit 1). The SI for the placebo treatment group is significantly different (P < 0·05) at visits 4 and 5 to that at baseline (visit 1).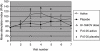
 ) and active (□) treatment groups is shown for each visit. The stimulation index (SI) for the active treatment group is significantly different (P < 0·05) at visits 3 and 4 to that at baseline (visit 1). The SI for the placebo treatment group is significantly different (P < 0·05) at visit 6 to that at baseline (visit 1).
) and active (□) treatment groups is shown for each visit. The stimulation index (SI) for the active treatment group is significantly different (P < 0·05) at visits 3 and 4 to that at baseline (visit 1). The SI for the placebo treatment group is significantly different (P < 0·05) at visit 6 to that at baseline (visit 1).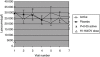
 ) and active (□) treatment groups are shown for each visit.
) and active (□) treatment groups are shown for each visit.Similar articles
-
Haemophilus influenzae and smoking-related obstructive airways disease.Int J Chron Obstruct Pulmon Dis. 2011;6:345-51. doi: 10.2147/COPD.S19359. Epub 2011 Jun 16. Int J Chron Obstruct Pulmon Dis. 2011. PMID: 21760721 Free PMC article.
-
Lung mucosal immunity to NTHi vaccine antigens: Antibodies in sputum of chronic obstructive pulmonary disease patients.Hum Vaccin Immunother. 2024 Dec 31;20(1):2343544. doi: 10.1080/21645515.2024.2343544. Epub 2024 Apr 24. Hum Vaccin Immunother. 2024. PMID: 38655676 Free PMC article. Clinical Trial.
-
Local and systemic antibody levels against protein D of Haemophilus influenzae following immunization and infection in rats.APMIS. 1996 Oct;104(10):709-17. APMIS. 1996. PMID: 8980621
-
Towards a vaccine for chronic obstructive pulmonary disease.Intern Med J. 2012 Jun;42(6):607-13. doi: 10.1111/j.1445-5994.2012.02752.x. Intern Med J. 2012. PMID: 22372964 Review.
-
An Oral Whole-Cell Killed Nontypeable Haemophilus influenzae Immunotherapeutic For The Prevention Of Acute Exacerbations Of Chronic Airway Disease.Int J Chron Obstruct Pulmon Dis. 2019 Oct 25;14:2423-2431. doi: 10.2147/COPD.S217317. eCollection 2019. Int J Chron Obstruct Pulmon Dis. 2019. PMID: 31695359 Free PMC article. Review.
Cited by
-
Haemophilus influenzae and smoking-related obstructive airways disease.Int J Chron Obstruct Pulmon Dis. 2011;6:345-51. doi: 10.2147/COPD.S19359. Epub 2011 Jun 16. Int J Chron Obstruct Pulmon Dis. 2011. PMID: 21760721 Free PMC article.
-
Changes in the prevalence and biofilm formation of Haemophilus influenzae and Haemophilus parainfluenzae from the respiratory microbiota of patients with sarcoidosis.BMC Infect Dis. 2016 Aug 26;16(1):449. doi: 10.1186/s12879-016-1793-7. BMC Infect Dis. 2016. PMID: 27562460 Free PMC article.
-
Influenza and pneumococcal vaccinations for patients with chronic obstructive pulmonary disease (COPD): an evidence-based review.Ont Health Technol Assess Ser. 2012;12(3):1-64. Epub 2012 Mar 1. Ont Health Technol Assess Ser. 2012. PMID: 23074431 Free PMC article. Review.
-
Haemophilus influenzae oral vaccination for preventing acute exacerbations of chronic bronchitis and chronic obstructive pulmonary disease.Cochrane Database Syst Rev. 2017 Jun 19;6(6):CD010010. doi: 10.1002/14651858.CD010010.pub3. Cochrane Database Syst Rev. 2017. PMID: 28626902 Free PMC article.
-
Bronchiectasis in Children: Current Concepts in Immunology and Microbiology.Front Pediatr. 2017 May 29;5:123. doi: 10.3389/fped.2017.00123. eCollection 2017. Front Pediatr. 2017. PMID: 28611970 Free PMC article. Review.
References
-
- Global Initiative for COPD: Global Strategy for the Diagnosis, Management and Prevention of COPD, 2006. Available at: http://www.goldcopd.com (updated 2009)
-
- Sethi S. Coinfection in exacerbations of COPD: a new frontier. Chest. 2006;129:223–4. - PubMed
-
- Sethi S. Bacteria infection and the pathogenesis of COPD. Chest. 2000;117:286–91. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

