Altered longevity-assurance activity of p53:p44 in the mouse causes memory loss, neurodegeneration and premature death
- PMID: 20409077
- PMCID: PMC2848983
- DOI: 10.1111/j.1474-9726.2010.00547.x
Altered longevity-assurance activity of p53:p44 in the mouse causes memory loss, neurodegeneration and premature death
Abstract
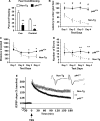
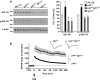
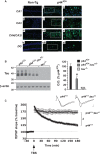
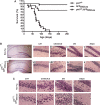
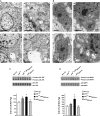
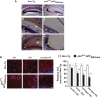
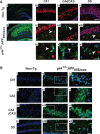
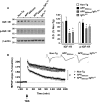
The longevity-assurance activity of the tumor suppressor p53 depends on the levels of Delta40p53 (p44), a short and naturally occurring isoform of the p53 gene. As such, increased dosage of p44 in the mouse leads to accelerated aging and short lifespan. Here we show that mice homozygous for a transgene encoding p44 (p44(+/+)) display cognitive decline and synaptic impairment early in life. The synaptic deficits are attributed to hyperactivation of insulin-like growth factor 1 receptor (IGF-1R) signaling and altered metabolism of the microtubule-binding protein tau. In fact, they were rescued by either Igf1r or Mapt haploinsufficiency. When expressing a human or a 'humanized' form of the amyloid precursor protein (APP), p44(+/+) animals developed a selective degeneration of memory-forming and -retrieving areas of the brain, and died prematurely. Mechanistically, the neurodegeneration was caused by both paraptosis- and autophagy-like cell deaths. These results indicate that altered longevity-assurance activity of p53:p44 causes memory loss and neurodegeneration by affecting IGF-1R signaling. Importantly, Igf1r haploinsufficiency was also able to correct the synaptic deficits of APP(695/swe) mice, a model of Alzheimer's disease.
Figures


References
-
- Auer T, Barsi P, Bone B, Angyalosi A, Aradi M, Szalay C, Horvath RA, Kovacs N, Kotek G, Fogarasi A, Komoly S, Janszky I, Schwarcz A, Janszky J. History of simple febrile seizures is associated with hippocampal abnormalities in adults. Epilepsia. 2008;49:1562–1569. - PubMed
-
- Bancher C, Brunner C, Lassmann H, Budka H, Jellinger K, Wiche G, Seitelberger F, Grundke-Iqbal I, Iqbal K, Wisniewski HM. Accumulation of abnormally phosphorylated tau precedes the formation of neurofibrillary tangles in Alzheimer’s disease. Brain Res. 1989;477:90–99. - PubMed
-
- Borchelt DR, Thinakaran G, Eckman CB, Lee MK, Davenport F, Ratovitsky T, Prada CM, Kim G, Seekins S, Yager D, Slunt HH, Wang R, Seeger M, Levey AI, Gandy SE, Copeland NG, Jenkins NA, Price DL, Younkin SG, Sisodia SS. Familial Alzheimer’s disease-linked presenilin 1 variants elevate Abeta1-42/1-40 ratio in vitro and in vivo. Neuron. 1996;17:1005–1013. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

