Efficacy of live attenuated influenza vaccine in children 6 months to 17 years of age
- PMID: 20409210
- PMCID: PMC4941661
- DOI: 10.1111/j.1750-2659.2009.00124.x
Efficacy of live attenuated influenza vaccine in children 6 months to 17 years of age
Abstract
Background: It has been suggested that live attenuated influenza vaccine (LAIV) may be less effective in older individuals because of prior wild-type influenza infections. LAIV is currently approved in the United States, South Korea and Hong Kong for individuals 2-49 years of age.
Objective: To examine data from previously published pediatric studies to determine the efficacy of LAIV in various age groups.
Methods: Four studies in which the subject age range exceeded 36 months were identified: one 2-year study comparing LAIV with placebo and three 1-year studies comparing LAIV with trivalent inactivated influenza vaccine (TIV). Efficacy against any strain regardless of antigenic similarity to vaccine was analyzed by age; age groups were based on the study design and sample size. A logistic regression model was used to assess whether age, as a continuous variable, was an effect modifier on LAIV efficacy.
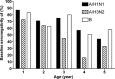
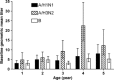
Results: The efficacy of LAIV did not vary with age in children aged 15-84 months compared with placebo or in children aged 6 months to 17 years compared with TIV.
Conclusions: The available data from prospective, randomized studies in children does not support the concept that prior repeated exposure to influenza, either through wild-type infection or vaccination with live, attenuated or inactivated vaccines, reduces the efficacy of LAIV compared with placebo or TIV. The decreased immunologic responses to LAIV reported in older individuals or those with pre-existing immunity do not appear to translate into reduced protection from influenza in children.
Figures
References
-
- Ashkenazi S, Vertruyen A, Aristegui J et al. Superior relative efficacy of live attenuated influenza vaccine compared with inactivated influenza vaccine in young children with recurrent respiratory tract infections. Pediatr Infect Dis J 2006; 25:870–879. - PubMed
-
- Rhorer J, Ambrose CS, Dickinson S et al. Efficacy of live attenuated influenza vaccine in children: a meta‐analysis of nine randomized clinical trials. Vaccine 2009; 27:1101–1110. - PubMed
-
- Belshe RB, Edwards KM, Vesikari T et al. Live attenuated versus inactivated influenza vaccine in infants and young children. N Engl J Med 2007; 356:685–696. - PubMed
-
- Belshe RB, Gruber WC, Mendelman PM et al. Efficacy of vaccination with live attenuated, cold‐adapted, trivalent, intranasal influenza virus vaccine against a variant (A/Sydney) not contained in the vaccine. J Pediatr 2000; 136:168–175. - PubMed
-
- Belshe RB, Mendelman PM, Treanor J et al. The efficacy of live attenuated, cold‐adapted, trivalent, intranasal influenzavirus vaccine in children. N Engl J Med 1998; 338:1405–1412. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical