The lung cancer exercise training study: a randomized trial of aerobic training, resistance training, or both in postsurgical lung cancer patients: rationale and design
- PMID: 20409311
- PMCID: PMC2888787
- DOI: 10.1186/1471-2407-10-155
The lung cancer exercise training study: a randomized trial of aerobic training, resistance training, or both in postsurgical lung cancer patients: rationale and design
Abstract
Background: The Lung Cancer Exercise Training Study (LUNGEVITY) is a randomized trial to investigate the efficacy of different types of exercise training on cardiorespiratory fitness (VO2peak), patient-reported outcomes, and the organ components that govern VO2peak in post-operative non-small cell lung cancer (NSCLC) patients.
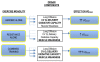
Methods/design: Using a single-center, randomized design, 160 subjects (40 patients/study arm) with histologically confirmed stage I-IIIA NSCLC following curative-intent complete surgical resection at Duke University Medical Center (DUMC) will be potentially eligible for this trial. Following baseline assessments, eligible participants will be randomly assigned to one of four conditions: (1) aerobic training alone, (2) resistance training alone, (3) the combination of aerobic and resistance training, or (4) attention-control (progressive stretching). The ultimate goal for all exercise training groups will be 3 supervised exercise sessions per week an intensity above 70% of the individually determined VO2peak for aerobic training and an intensity between 60 and 80% of one-repetition maximum for resistance training, for 30-45 minutes/session. Progressive stretching will be matched to the exercise groups in terms of program length (i.e., 16 weeks), social interaction (participants will receive one-on-one instruction), and duration (30-45 mins/session). The primary study endpoint is VO2peak. Secondary endpoints include: patient-reported outcomes (PROs) (e.g., quality of life, fatigue, depression, etc.) and organ components of the oxygen cascade (i.e., pulmonary function, cardiac function, skeletal muscle function). All endpoints will be assessed at baseline and postintervention (16 weeks). Substudies will include genetic studies regarding individual responses to an exercise stimulus, theoretical determinants of exercise adherence, examination of the psychological mediators of the exercise - PRO relationship, and exercise-induced changes in gene expression.
Discussion: VO2peak is becoming increasingly recognized as an outcome of major importance in NSCLC. LUNGEVITY will identify the optimal form of exercise training for NSCLC survivors as well as provide insight into the physiological mechanisms underlying this effect. Overall, this study will contribute to the establishment of clinical exercise therapy rehabilitation guidelines for patients across the entire NSCLC continuum.
Trial registration: NCT00018255.
Figures
References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous