MicroRNA-184 inhibits neuroblastoma cell survival through targeting the serine/threonine kinase AKT2
- PMID: 20409325
- PMCID: PMC2864218
- DOI: 10.1186/1476-4598-9-83
MicroRNA-184 inhibits neuroblastoma cell survival through targeting the serine/threonine kinase AKT2
Abstract
Background: Neuroblastoma is a paediatric cancer of the sympathetic nervous system. The single most important genetic indicator of poor clinical outcome is amplification of the MYCN transcription factor. One of many down-stream MYCN targets is miR-184, which is either directly or indirectly repressed by this transcription factor, possibly due to its pro-apoptotic effects when ectopically over-expressed in neuroblastoma cells. The purpose of this study was to elucidate the molecular mechanism by which miR-184 conveys pro-apoptotic effects.
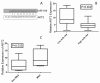
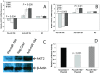
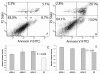
Results: We demonstrate that the knock-down of endogenous miR-184 has the opposite effect of ectopic up-regulation, leading to enhanced neuroblastoma cell numbers. As a mechanism of how miR-184 causes apoptosis when over-expressed, and increased cell numbers when inhibited, we demonstrate direct targeting and degradation of AKT2, a major downstream effector of the phosphatidylinositol 3-kinase (PI3K) pathway, one of the most potent pro-survival pathways in cancer. The pro-apoptotic effects of miR-184 ectopic over-expression in neuroblastoma cell lines is reproduced by siRNA inhibition of AKT2, while a positive effect on cell numbers similar to that obtained by the knock-down of endogenous miR-184 can be achieved by ectopic up-regulation of AKT2. Moreover, co-transfection of miR-184 with an AKT2 expression vector lacking the miR-184 target site in the 3'UTR rescues cells from the pro-apoptotic effects of miR-184.
Conclusions: MYCN contributes to tumorigenesis, in part, by repressing miR-184, leading to increased levels of AKT2, a direct target of miR-184. Thus, two important genes with positive effects on cell growth and survival, MYCN and AKT2, can be linked into a common genetic pathway through the actions of miR-184. As an inhibitor of AKT2, miR-184 could be of potential benefit in miRNA mediated therapeutics of MYCN amplified neuroblastoma and other forms of cancer.
Figures



References
-
- Alaminos M, Mora J, Cheung NK, Smith A, Qin J, Chen L, Gerald WL. Genome-wide analysis of gene expression associated with MYCN in human neuroblastoma. Cancer Res. 2003;63:4538–4546. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

