Effects of pneumococcal conjugate vaccine 2 years after its introduction, the Netherlands
- PMID: 20409372
- PMCID: PMC2953990
- DOI: 10.3201/eid1605.091223
Effects of pneumococcal conjugate vaccine 2 years after its introduction, the Netherlands
Abstract
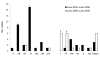
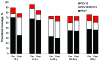
In the Netherlands, the 7-valent pneumococcal conjugate vaccine (PCV-7) was implemented in a 3+1-dose schedule in the national immunization program for infants born after April 1, 2006. To assess the vaccine's effectiveness, we compared disease incidence before and after vaccine implementation (June 2004-June 2006 and June 2006-June 2008, respectively). We serotyped 2,552 invasive pneumococcal isolates from throughout the Netherlands, covering 25% of the country's population. Clinical characteristics were extracted from hospital records. After June 2006, vaccine-serotype invasive pneumococcal disease (IPD) decreased 90% (95% confidence interval [CI] 68%-97%) in children age eligible for PCV-7; simultaneously, however, non-vaccine-serotype IPD increased by 71% (not significant), resulting in a 44% total net IPD reduction (95% CI 7%-66%). IPD rates did not change for other age groups. In the Netherlands, PCV-7 offered high protection against vaccine-serotype IPD in vaccinated children, but increases of non-vaccine-serotype IPD reduced net vaccine benefits.
Figures

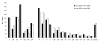

References
-
- Centers for Disease Control and Prevention. Invasive pneumococcal disease in children 5 years after conjugate vaccine introduction—eight states, 1998–2005. MMWR Morb Mortal Wkly Rep. 2008;57:144–8. - PubMed
-
- Pelton SI, Huot H, Finkelstein JA, Bishop CJ, Hsu KK, Kellenberg J, et al. Emergence of 19A as virulent and multidrug resistant pneumococcus in Massachusetts following universal immunization of infants with pneumococcal conjugate vaccine. Pediatr Infect Dis J. 2007;26:468–72. 10.1097/INF.0b013e31803df9ca - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
