Mechano-chemical feedbacks regulate actin mesh growth in lamellipodial protrusions
- PMID: 20409456
- PMCID: PMC2856144
- DOI: 10.1016/j.bpj.2009.11.054
Mechano-chemical feedbacks regulate actin mesh growth in lamellipodial protrusions
Abstract
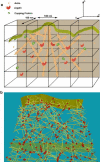
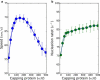
During cell motion on a substratum, eukaryotic cells project sheetlike lamellipodia which contain a dynamically remodeling three-dimensional actin mesh. A number of regulatory proteins and subtle mechano-chemical couplings determine the lamellipodial protrusion dynamics. To study these processes, we constructed a microscopic physico-chemical computational model, which incorporates a number of fundamental reaction and diffusion processes, treated in a fully stochastic manner. Our work sheds light on the way lamellipodial protrusion dynamics is affected by the concentrations of actin and actin-binding proteins. In particular, we found that protrusion speed saturates at very high actin concentrations, where filament nucleation does not keep up with protrusion. This results in sparse filamentous networks, and, consequently, high resistance forces on individual filaments. We also observed maxima in lamellipodial growth rates as a function of Arp2/3, a nucleating protein, and capping proteins. We provide detailed physical explanations behind these effects. In particular, our work supports the actin-funneling-hypothesis explanation of protrusion speed enhancement at low capping protein concentrations. Our computational results are in agreement with a number of related experiments. Overall, our work emphasizes that elongation and nucleation processes work highly cooperatively in determining the optimal protrusion speed for the actin mesh in lamellipodia.
Copyright 2010 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures






Similar articles
-
How does the antagonism between capping and anti-capping proteins affect actin network dynamics?J Phys Condens Matter. 2011 Sep 21;23(37):374101. doi: 10.1088/0953-8984/23/37/374101. Epub 2011 Aug 23. J Phys Condens Matter. 2011. PMID: 21862844
-
Arp2/3 complex is essential for actin network treadmilling as well as for targeting of capping protein and cofilin.Mol Biol Cell. 2013 Sep;24(18):2861-75. doi: 10.1091/mbc.E12-12-0857. Epub 2013 Jul 24. Mol Biol Cell. 2013. PMID: 23885122 Free PMC article.
-
Efficiency of lamellipodia protrusion is determined by the extent of cytosolic actin assembly.Mol Biol Cell. 2017 May 15;28(10):1311-1325. doi: 10.1091/mbc.E16-05-0334. Epub 2017 Mar 22. Mol Biol Cell. 2017. PMID: 28331069 Free PMC article.
-
Steering cell migration: lamellipodium dynamics and the regulation of directional persistence.Nat Rev Mol Cell Biol. 2014 Sep;15(9):577-90. doi: 10.1038/nrm3861. Nat Rev Mol Cell Biol. 2014. PMID: 25145849 Review.
-
Actin dynamics in cell migration.Essays Biochem. 2019 Oct 31;63(5):483-495. doi: 10.1042/EBC20190015. Essays Biochem. 2019. PMID: 31551324 Free PMC article. Review.
Cited by
-
Quantifying dissipation in actomyosin networks.Interface Focus. 2019 Jun 6;9(3):20180078. doi: 10.1098/rsfs.2018.0078. Epub 2019 Apr 19. Interface Focus. 2019. PMID: 31065344 Free PMC article.
-
Modeling cell protrusion predicts how myosin II and actin turnover affect adhesion-based signaling.Biophys J. 2022 Jan 4;121(1):102-118. doi: 10.1016/j.bpj.2021.11.2889. Epub 2021 Dec 1. Biophys J. 2022. PMID: 34861242 Free PMC article.
-
Control of polarized assembly of actin filaments in cell motility.Cell Mol Life Sci. 2015 Aug;72(16):3051-67. doi: 10.1007/s00018-015-1914-2. Epub 2015 May 7. Cell Mol Life Sci. 2015. PMID: 25948416 Free PMC article. Review.
-
Building a dendritic actin filament network branch by branch: models of filament orientation pattern and force generation in lamellipodia.Biophys Rev. 2018 Dec;10(6):1577-1585. doi: 10.1007/s12551-018-0475-7. Epub 2018 Nov 12. Biophys Rev. 2018. PMID: 30421277 Free PMC article. Review.
-
How capping protein enhances actin filament growth and nucleation on biomimetic beads.Phys Biol. 2015 Nov 25;12(6):066008. doi: 10.1088/1478-3975/12/6/066008. Phys Biol. 2015. PMID: 26602226 Free PMC article.
References
-
- Alberts B., Johnson A., Walter P. 4th Ed. Garland Science; New York: 2002. Molecular Biology of the Cell.
-
- Lauffenburger D.A., Horwitz A.F. Cell migration: a physically integrated molecular process. Cell. 1996;84:359–369. - PubMed
-
- Theriot J.A., Mitchison T.J. Actin microfilament dynamics in locomoting cells. Nature. 1991;352:126–131. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

