Design of active transport must be highly intricate: a possible role of myosin and Ena/VASP for G-actin transport in filopodia
- PMID: 20409462
- PMCID: PMC2856189
- DOI: 10.1016/j.bpj.2009.12.4325
Design of active transport must be highly intricate: a possible role of myosin and Ena/VASP for G-actin transport in filopodia
Abstract
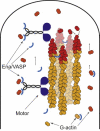
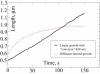
Recent modeling of filopodia--the actin-based cell organelles employed for sensing and motility--reveals that one of the key limiting factors of filopodial length is diffusional transport of G-actin monomers to the polymerizing barbed ends. We have explored the possibility of active transport of G-actin by myosin motors, which would be an expected biological response to overcome the limitation of a diffusion-based process. We found that in a straightforward implementation of active transport the increase in length was unimpressive, < or = 30%, due to sequestering of G-actin by freely diffusing motors. However, artificially removing motor sequestration reactions led to approximately threefold increases in filopodial length, with the transport being mainly limited by the motors failing to detach from the filaments near the tip, clogging the cooperative conveyer belt dynamics. Making motors sterically transparent led to a qualitative change of the dynamics to a different regime of steady growth without a stationary length. Having identified sequestration and clogging as ubiquitous constraints to motor-driven transport, we devised and tested a speculative means to sidestep these limitations in filopodia by employing cross-linking and putative scaffolding roles of Ena/VASP proteins. We conclude that a naïve design of molecular-motor-based active transport would almost always be inefficient--an intricately organized kinetic scheme, with finely tuned rate constants, is required to achieve high-flux transport.
Copyright 2010 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures



Similar articles
-
Ena/VASP proteins have an anti-capping independent function in filopodia formation.Mol Biol Cell. 2007 Jul;18(7):2579-91. doi: 10.1091/mbc.e06-11-0990. Epub 2007 May 2. Mol Biol Cell. 2007. PMID: 17475772 Free PMC article.
-
Protein fluxes along the filopodium as a framework for understanding the growth-retraction dynamics: the interplay between diffusion and active transport.Cell Adh Migr. 2011 Sep-Oct;5(5):448-56. doi: 10.4161/cam.5.5.17868. Cell Adh Migr. 2011. PMID: 21975554 Free PMC article.
-
VASP-mediated actin dynamics activate and recruit a filopodia myosin.Elife. 2021 May 27;10:e68082. doi: 10.7554/eLife.68082. Elife. 2021. PMID: 34042588 Free PMC article.
-
Formins and VASPs may co-operate in the formation of filopodia.Biochem Soc Trans. 2005 Dec;33(Pt 6):1256-9. doi: 10.1042/BST0331256. Biochem Soc Trans. 2005. PMID: 16246092 Review.
-
Ena/VASP proteins: regulators of the actin cytoskeleton and cell migration.Annu Rev Cell Dev Biol. 2003;19:541-64. doi: 10.1146/annurev.cellbio.19.050103.103356. Annu Rev Cell Dev Biol. 2003. PMID: 14570581 Review.
Cited by
-
Robust patterns in the stochastic organization of filopodia.BMC Cell Biol. 2010 Nov 17;11:86. doi: 10.1186/1471-2121-11-86. BMC Cell Biol. 2010. PMID: 21083909 Free PMC article.
-
Myosin X is recruited to nascent focal adhesions at the leading edge and induces multi-cycle filopodial elongation.Sci Rep. 2017 Oct 20;7(1):13685. doi: 10.1038/s41598-017-06147-6. Sci Rep. 2017. PMID: 29057977 Free PMC article.
-
Multi-Grid Reaction-Diffusion Master Equation: Applications to Morphogen Gradient Modelling.Bull Math Biol. 2024 Nov 27;87(1):6. doi: 10.1007/s11538-024-01377-y. Bull Math Biol. 2024. PMID: 39601934 Free PMC article.
-
Remarkable structural transformations of actin bundles are driven by their initial polarity, motor activity, crosslinking, and filament treadmilling.PLoS Comput Biol. 2019 Jul 9;15(7):e1007156. doi: 10.1371/journal.pcbi.1007156. eCollection 2019 Jul. PLoS Comput Biol. 2019. PMID: 31287817 Free PMC article.
-
Physical model for the geometry of actin-based cellular protrusions.Biophys J. 2014 Aug 5;107(3):576-587. doi: 10.1016/j.bpj.2014.05.040. Biophys J. 2014. PMID: 25099797 Free PMC article.
References
-
- Lorenz M., Yamaguchi H., Condeelis J. Imaging sites of N-wasp activity in lamellipodia and invadopodia of carcinoma cells. Curr. Biol. 2004;14:697–703. - PubMed
-
- Dent E.W., Gertler F.B. Cytoskeletal dynamics and transport in growth cone motility and axon guidance. Neuron. 2003;40:209–227. - PubMed
-
- Noselli S. Drosophila, actin and videotape—new insights in wound healing. Nat. Cell Biol. 2002;4:E251–E253. - PubMed
-
- Lawson N.D., Weinstein B.M. In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev. Biol. 2002;248:307–318. - PubMed
-
- Pollard T.D., Borisy G.G. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112:453–465. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

