Role of the transmembrane potential in the membrane proton leak
- PMID: 20409469
- PMCID: PMC2856137
- DOI: 10.1016/j.bpj.2009.12.4301
Role of the transmembrane potential in the membrane proton leak
Abstract
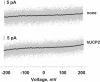
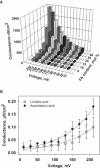
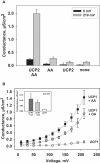
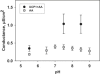
The molecular mechanism responsible for the regulation of the mitochondrial membrane proton conductance (G) is not clearly understood. This study investigates the role of the transmembrane potential (DeltaPsim) using planar membranes, reconstituted with purified uncoupling proteins (UCP1 and UCP2) and/or unsaturated FA. We show that high DeltaPsim (similar to DeltaPsim in mitochondrial State IV) significantly activates the protonophoric function of UCPs in the presence of FA. The proton conductance increases nonlinearly with DeltaPsim. The application of DeltaPsim up to 220 mV leads to the overriding of the protein inhibition at a constant ATP concentration. Both, the exposure of FA-containing bilayers to high DeltaPsim and the increase of FA membrane concentration bring about the significant exponential Gm increase, implying the contribution of FA in proton leak. Quantitative analysis of the energy barrier for the transport of FA anions in the presence and absence of protein suggests that FA- remain exposed to membrane lipids while crossing the UCP-containing membrane. We believe this study shows that UCPs and FA decrease DeltaPsim more effectively if it is sufficiently high. Thus, the tight regulation of proton conductance and/or FA concentration by DeltaPsim may be key in mitochondrial respiration and metabolism.
Copyright 2010 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures
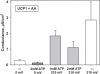
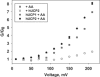
Similar articles
-
Fatty acids are key in 4-hydroxy-2-nonenal-mediated activation of uncoupling proteins 1 and 2.PLoS One. 2013 Oct 28;8(10):e77786. doi: 10.1371/journal.pone.0077786. eCollection 2013. PLoS One. 2013. PMID: 24204965 Free PMC article.
-
The regulation and physiology of mitochondrial proton leak.Physiology (Bethesda). 2011 Jun;26(3):192-205. doi: 10.1152/physiol.00046.2010. Physiology (Bethesda). 2011. PMID: 21670165 Review.
-
Polyunsaturated fatty acids activate human uncoupling proteins 1 and 2 in planar lipid bilayers.FASEB J. 2007 Apr;21(4):1137-44. doi: 10.1096/fj.06-7489com. Epub 2007 Jan 22. FASEB J. 2007. PMID: 17242157
-
Reconstitution of recombinant uncoupling proteins: UCP1, -2, and -3 have similar affinities for ATP and are unaffected by coenzyme Q10.J Biol Chem. 2003 Jul 11;278(28):25825-31. doi: 10.1074/jbc.M302126200. Epub 2003 May 6. J Biol Chem. 2003. PMID: 12734183
-
UCP1: A transporter for H+ and fatty acid anions.Biochimie. 2017 Mar;134:28-34. doi: 10.1016/j.biochi.2016.10.013. Epub 2016 Oct 27. Biochimie. 2017. PMID: 27984203 Free PMC article. Review.
Cited by
-
Combined Recognition Imaging and Force Spectroscopy: A New Mode for Mapping and Studying Interaction Sites at Low Lateral Density.Sci Adv Mater. 2017 Jan 1;9(1):128-134. doi: 10.1166/sam.2017.3066. Sci Adv Mater. 2017. PMID: 29743989 Free PMC article.
-
Mitochondrial Uncoupling Proteins (UCP1-UCP3) and Adenine Nucleotide Translocase (ANT1) Enhance the Protonophoric Action of 2,4-Dinitrophenol in Mitochondria and Planar Bilayer Membranes.Biomolecules. 2021 Aug 9;11(8):1178. doi: 10.3390/biom11081178. Biomolecules. 2021. PMID: 34439844 Free PMC article.
-
Test systems to study the structure and function of uncoupling protein 1: a critical overview.Front Endocrinol (Lausanne). 2011 Nov 8;2:63. doi: 10.3389/fendo.2011.00063. eCollection 2011. Front Endocrinol (Lausanne). 2011. PMID: 22654819 Free PMC article.
-
AFM-Based Force Spectroscopy Guided by Recognition Imaging: A New Mode for Mapping and Studying Interaction Sites at Low Lateral Density.Methods Protoc. 2019 Jan 8;2(1):6. doi: 10.3390/mps2010006. Methods Protoc. 2019. PMID: 31164590 Free PMC article.
-
Effects of voluntary exercise on the expression of browning markers in visceral and subcutaneous fat tissue of normotensive and spontaneously hypertensive rats.Pflugers Arch. 2022 Feb;474(2):205-215. doi: 10.1007/s00424-021-02629-9. Epub 2021 Dec 10. Pflugers Arch. 2022. PMID: 34893937 Free PMC article.
References
-
- Nicholls D.G. A history of UCP1. Biochem. Soc. Trans. 2001;29:751–755. - PubMed
-
- Nedergaard J., Golozoubova V., Cannon B. UCP1: the only protein able to mediate adaptive non-shivering thermogenesis and metabolic inefficiency. Biochim. Biophys. Acta. 2001;1504:82–106. - PubMed
-
- Skulachev V.P. Uncoupling: new approaches to an old problem of bioenergetics. Biochim. Biophys. Acta. 1998;1363:100–124. - PubMed
-
- Dlasková A., Hlavatá L., Jezek P. Oxidative stress caused by blocking of mitochondrial complex I H(+) pumping as a link in aging/disease vicious cycle. Int. J. Biochem. Cell Biol. 2008;40:1792–1805. - PubMed
-
- Brand M.D., Affourtit C., Parker N. Mitochondrial superoxide: production, biological effects, and activation of uncoupling proteins. Free Radic. Biol. Med. 2004;37:755–767. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

