Nitric oxide is elicited and inhibits viral replication in pigs infected with porcine respiratory coronavirus but not porcine reproductive and respiratory syndrome virus
- PMID: 20409593
- PMCID: PMC2902704
- DOI: 10.1016/j.vetimm.2010.03.022
Nitric oxide is elicited and inhibits viral replication in pigs infected with porcine respiratory coronavirus but not porcine reproductive and respiratory syndrome virus
Abstract
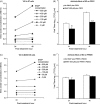
There is little information on the role of nitric oxide (NO) in innate immunity to respiratory coronavirus (CoV) infections. We examined NO levels by Greiss assay in bronchoalveolar lavage (BAL) of pigs infected with either porcine respiratory coronavirus (PRCV) or porcine reproductive and respiratory syndrome virus (PRRSV), a member of Nidovirales, like CoV. The antiviral effects of NO on these two viruses were tested in an in vitro system using a NO donor, S-nitroso-N-acetylpenicillamine (SNAP). We detected a large increase in NO levels in BAL fluids of PRCV-infected pigs, but not in PRRSV-infected pigs. Pulmonary epithelial cell necrosis induced by PRCV coincided with increased NO. Moreover, NO levels in cell culture medium of PRRSV-infected alveolar macrophages (AMs) did not differ from that of mock-infected AMs. Antiviral assays showed that NO significantly inhibited PRCV replication in swine testicular (ST) cells, whereas PRRSV was not susceptible to NO based on the conditions tested. Our study suggests that unlike PRRSV which induces apoptosis in AMs, respiratory CoVs such as PRCV that infect pulmonary epithelial cells and cause cytolysis, induce NO production in the respiratory tract. Thus, NO may play a role in innate immunity to respiratory CoV infections by inhibiting viral replication.
Copyright 2010 Elsevier B.V. All rights reserved.
Figures

References
-
- Costers S., Lefebvre D.J., Delputte P.L., Nauwynck H.J. Porcine reproductive and respiratory syndrome virus modulates apoptosis during replication in alveolar macrophages. Arch. Virol. 2008;153:1453–1465. - PubMed
-
- Davis I., Matalon S. Reactive species in viral pneumonitis: lessons from animal models. News Physiol. Sci. 2001;16:185–190. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

