Lipoteichoic acid from Staphylococcus aureus exacerbates respiratory disease in porcine respiratory coronavirus-infected pigs
- PMID: 20409735
- PMCID: PMC2932768
- DOI: 10.1016/j.tvjl.2010.03.001
Lipoteichoic acid from Staphylococcus aureus exacerbates respiratory disease in porcine respiratory coronavirus-infected pigs
Abstract
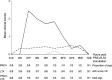
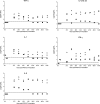
The objective of this study was to assess if lipoteichoic acid (LTA), produced by Staphylococcus aureus, exacerbates respiratory disease in porcine respiratory coronavirus (PRCV)-infected pigs, as has previously been shown with lipopolysaccharide. Piglets were inoculated with PRCV and 24h later with S. aureus LTA. Clinical signs, lung virus titres, inflammatory cells and cytokines in bronchoalveolar lavage fluid (BALF) were compared with those of animals in PRCV- and LTA-inoculated control groups. All PRCV-LTA-inoculated pigs except one developed severe respiratory disease, whereas clinical signs in the control groups were minimal or absent. Virus titres and grossly visible pulmonary lesions were similar in the PRCV-LTA- and PRCV-inoculated groups and were not detected in the LTA group. Neutrophil percentages in BALF were higher in the PRCV-LTA than in the PRCV group. There was no significant difference in interferon (IFN)-γ, interleukin (IL)-1, IL-6, IL-12/IL-23 and tumour necrosis factor (TNF)-α concentrations in BALF between the PRCV-LTA and PRCV groups, but levels of IL-6, IL-12/IL-23 and IFN-γ were higher in the PRCV-LTA-inoculated than in the LTA-inoculated controls. The findings suggest that the experimentally-induced respiratory disease was not mediated by cytokine over-production, but rather reflected the concerted action of particular cytokine interactions and/or as yet unidentified mediators. This is the first in vivo study to report the synergistic interaction between a virus and LTA in enhancing the severity of respiratory disease in the pig. Given that Gram-positive bacteria, capable of producing LTA, are commonly found in pig accommodation, the role of this compound in the development of the porcine respiratory disease complex requires further investigation.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures
References
-
- Atanasova K., Van Gucht S., Barbé F., Lefebvre D., Chiers K., Van Reeth K. Lung cell tropism and inflammatory cytokine-profile of porcine respiratory coronavirus infection. Open Veterinary Science Journal. 2008;2:117–126.
-
- Bastos K., Marinho C., Barboza R., Russo M., Alvarez J., D’Imperio Lima M. What kind of message does IL-12/IL-23 bring to macrophages and dendritic cells? Microbes and Infection. 2004;6:630–636. - PubMed
-
- Brockmeier S., Halbur P., Thacker E. Porcine respiratory disease complex. In: Brogden K., Guthmiller J., editors. Polymicrobial Diseases. American Society for Microbiology; Washington, DC: 2002. pp. 231–258.
-
- Christensen G., Sorensen V., Mousing J. Diseases of the respiratory system. In: Straw B., D’Allaire S., Mengeling W., Taylor D., editors. Diseases of Swine. Iowa State University Press; Ames, Iowa, USA: 1999. pp. 913–940.
-
- Crook B., Robertson J., Glass S., Botheroyd E., Lacey J., Topping M. Airborne dust, ammonia, microorganisms, and antigens in pig confinement houses and the respiratory health of exposed farm workers. American Industrial Hygiene Association Journal. 1991;52:271–279. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

