O-GlcNAcylation: a novel post-translational mechanism to alter vascular cellular signaling in health and disease: focus on hypertension
- PMID: 20409980
- PMCID: PMC3022480
- DOI: 10.1016/j.jash.2009.09.004
O-GlcNAcylation: a novel post-translational mechanism to alter vascular cellular signaling in health and disease: focus on hypertension
Abstract
O-Linked attachment of beta-N-acetyl-glucosamine (O-GlcNAc) on serine and threonine residues of nuclear and cytoplasmic proteins is a highly dynamic posttranslational modification that plays a key role in signal transduction pathways. Preliminary data show that O-GlcNAcylation may represent a key regulatory mechanism in the vasculature, modulating contractile and relaxant responses. Proteins with an important role in vascular function, such as endothelial nitric oxide synthase, sarcoplasmic reticulum Ca(2+)-ATPase, protein kinase C, mitogen-activated protein kinases, and proteins involved in cytoskeleton regulation and microtubule assembly are targets for O-GlcNAcylation, indicating that this posttranslational modification may play an important role in vascular reactivity. Here, we will focus on a few specific pathways that contribute to vascular function and cardiovascular disease-associated vascular dysfunction, and the implications of their modification by O-GlcNAc. New chemical tools have been developed to detect and study O-GlcNAcylation, including inhibitors of O-GlcNAc enzymes, chemoenzymatic tagging methods, and quantitative proteomics strategies; these will also be briefly addressed. An exciting challenge in the future will be to better understand the cellular dynamics of this posttranslational modification, as well as the signaling pathways and mechanisms by which O-GlcNAc is regulated on specific proteins in the vasculature in health and disease.
Figures


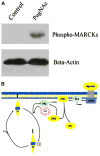
References
-
- Hancock JT. Cell signalling. England: Addison-Wesley; 1997.
-
- Gomperts BD, Kramer IM, Tatham PER. Signal transduction. San Diego: Academic Press; 2002.
-
- Webb RC. Smooth muscle contraction and relaxation. Adv Physiol Educ. 2003;27:201–6. - PubMed
-
- Tostes RC, Leite R, Webb RC. Vascular smooth muscle contraction and relaxation. In: Izzo JL, Sica D, Black HR, editors. Hypertension primer. The essentials of high blood pressure. 4. Chapter A10. Philadelphia: Lippincott Williams & Wilkins; 2007. pp. 34–7.
-
- Booz GW, Baker KM. Protein phosphorylation. In: Izzo JL, Sica D, Black HR, editors. Hypertension primer: The essentials of high blood pressure. 4. Chapter A5. Philadelphia: Lippincott Williams & Wilkins; 2007. pp. 16–21.
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

