Evidence from computer simulations for alterations in the membrane biophysical properties and dendritic processing of synaptic inputs in mutant superoxide dismutase-1 motoneurons
- PMID: 20410108
- PMCID: PMC4671367
- DOI: 10.1523/JNEUROSCI.0434-10.2010
Evidence from computer simulations for alterations in the membrane biophysical properties and dendritic processing of synaptic inputs in mutant superoxide dismutase-1 motoneurons
Abstract
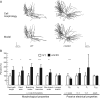
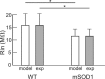
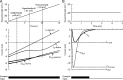
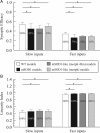
A critical step in improving our understanding of the development of amyotrophic lateral sclerosis (ALS) is to identify the factors contributing to the alterations in the excitability of motoneurons and assess their individual contributions. Here we investigated the early alterations in the passive electrical and morphological properties of neonatal spinal motoneurons that occur by 10 d after birth, long before disease onset. We identified some of the factors contributing to these alterations, and estimated their individual contributions. To achieve this goal, we undertook a computer simulation analysis using realistic morphologies of reconstructed wild-type (WT) and mutant superoxide dismutase-1 (mSOD1) motoneurons. Ion channel parameters of these models were then tuned to match the experimental data on electrical properties obtained from these same motoneurons. We found that the reduced excitability of mSOD1 models was accompanied with decreased specific membrane resistance by approximately 25% and efficacy of synaptic inputs (slow and fast) by 12-22%. Linearity of summation of synaptic currents was similar to WT. We also assessed the contribution of the alteration in dendritic morphology alone to this decreased excitability and found that it reduced the input resistance by 10% and the efficacy of synaptic inputs by 7-15%. Our results were also confirmed in models with dendritic active conductances. Our simulations indicated that the alteration in passive electrical properties of mSOD1 models resulted from concurrent alterations in their morphology and membrane biophysical properties, and consequently altered the motoneuronal dendritic processing of synaptic inputs. These results clarify new aspects of spinal motoneurons malfunction in ALS.
Figures





Similar articles
-
Inhibitory synaptic regulation of motoneurons: a new target of disease mechanisms in amyotrophic lateral sclerosis.Mol Neurobiol. 2012 Feb;45(1):30-42. doi: 10.1007/s12035-011-8217-x. Epub 2011 Nov 10. Mol Neurobiol. 2012. PMID: 22072396 Free PMC article. Review.
-
Postnatal electrical and morphological abnormalities in lumbar motoneurons from transgenic mouse models of amyotrophic lateral sclerosis.Arch Ital Biol. 2007 Nov;145(3-4):311-23. Arch Ital Biol. 2007. PMID: 18075124
-
Depressed excitability and ion currents linked to slow exocytotic fusion pore in chromaffin cells of the SOD1(G93A) mouse model of amyotrophic lateral sclerosis.Am J Physiol Cell Physiol. 2015 Jan 1;308(1):C1-19. doi: 10.1152/ajpcell.00272.2014. Epub 2014 Nov 5. Am J Physiol Cell Physiol. 2015. PMID: 25377090
-
Postnatal dendritic development in lumbar motoneurons in mutant superoxide dismutase 1 mouse model of amyotrophic lateral sclerosis.Neuroscience. 2012 May 3;209:144-54. doi: 10.1016/j.neuroscience.2012.01.046. Epub 2012 Feb 11. Neuroscience. 2012. PMID: 22387111
-
Integration of synaptic and intrinsic dendritic currents in cat spinal motoneurons.Brain Res Brain Res Rev. 2002 Oct;40(1-3):1-8. doi: 10.1016/s0165-0173(02)00183-2. Brain Res Brain Res Rev. 2002. PMID: 12589901 Review.
Cited by
-
Homeostatic dysregulation in membrane properties of masticatory motoneurons compared with oculomotor neurons in a mouse model for amyotrophic lateral sclerosis.J Neurosci. 2015 Jan 14;35(2):707-20. doi: 10.1523/JNEUROSCI.1682-14.2015. J Neurosci. 2015. PMID: 25589764 Free PMC article.
-
Altered postnatal maturation of electrical properties in spinal motoneurons in a mouse model of amyotrophic lateral sclerosis.J Physiol. 2011 May 1;589(Pt 9):2245-60. doi: 10.1113/jphysiol.2010.200659. Epub 2011 Feb 28. J Physiol. 2011. PMID: 21486770 Free PMC article.
-
Diminution of voltage threshold plays a key role in determining recruitment of oculomotor nucleus motoneurons during postnatal development.PLoS One. 2011;6(12):e28748. doi: 10.1371/journal.pone.0028748. Epub 2011 Dec 9. PLoS One. 2011. PMID: 22174887 Free PMC article.
-
Development of modified cable models to simulate accurate neuronal active behaviors.J Appl Physiol (1985). 2014 Dec 1;117(11):1243-61. doi: 10.1152/japplphysiol.00496.2014. Epub 2014 Oct 2. J Appl Physiol (1985). 2014. PMID: 25277743 Free PMC article.
-
Developing electrical properties of postnatal mouse lumbar motoneurons.Front Cell Neurosci. 2015 Sep 2;9:349. doi: 10.3389/fncel.2015.00349. eCollection 2015. Front Cell Neurosci. 2015. PMID: 26388736 Free PMC article.
References
-
- Amendola J, Durand J. Morphological differences between wild-type and transgenic superoxide dismutase 1 lumbar motoneurons in postnatal mice. J Comp Neurol. 2008;511:329–341. - PubMed
-
- Amendola J, Verrier B, Roubertoux P, Durand J. Altered sensorimotor development in a transgenic mouse model of amyotrophic lateral sclerosis. Eur J Neurosci. 2004;20:2822–2826. - PubMed
-
- Berger AJ, Bayliss DA, Viana F. Modulation of neonatal rat hypoglossal motoneuron excitability by serotonin. Neurosci Lett. 1992;143:164–168. - PubMed
-
- Bories C, Amendola J, Lamotte d'Incamps B, Durand J. Early electrophysiological abnormalities in lumbar motoneurons in a transgenic mouse model of amyotrophic lateral sclerosis. Eur J Neurosci. 2007;25:451–459. - PubMed
-
- Bruijn LI, Becher MW, Lee MK, Anderson KL, Jenkins NA, Copeland NG, Sisodia SS, Rothstein JD, Borchelt DR, Price DL, Cleveland DW. ALS-linked SOD1 mutant G85R mediates damage to astrocytes and promotes rapidly progressive disease with SOD1-containing inclusions. Neuron. 1997;18:327–338. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
