Cotrafficking of SV2 and synaptotagmin at the synapse
- PMID: 20410110
- PMCID: PMC2866018
- DOI: 10.1523/JNEUROSCI.4781-09.2010
Cotrafficking of SV2 and synaptotagmin at the synapse
Abstract
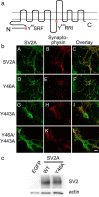
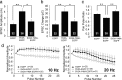
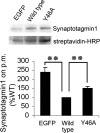
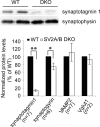
Synaptic vesicles are specialized cycling endosomes that contain a unique constellation of membrane proteins. Proteins are sorted to vesicles by short amino acid sequences that serve as binding sites for clathrin adaptor proteins. Here we show that a tyrosine-based endocytosis motif in the vesicle protein SV2 is required for trafficking to synaptic vesicles of both SV2 and the calcium sensor protein synaptotagmin. Aberrant neurotransmission in cultured hippocampal neurons lacking SV2 was rescued by expression of wild-type SV2A, but not by SV2A-Y46A, a mutant containing a disrupted endocytosis motif in SV2A's cytoplasmic N terminus. Neurons expressing SV2A-Y46A had significantly more SV2 on the plasma membrane, indicating reduced internalization. A screen for proteins that preferentially bound wild-type SV2A identified multiple endocytosis-related proteins, and in vitro binding studies confirmed binding to the clathrin adaptors AP2, EPS15, and amphiphysin 2/Bin1. Neurons lacking SV2 contained less synaptotagmin and had a higher proportion of synaptotagmin on the plasma membrane. Expression of either wild-type SV2A or SV2A-Y46A restored synaptotagmin expression levels; however, only wild-type SV2A restored a normal proportion of synaptotagmin on the plasma membrane. These findings indicate that SV2 influences the expression and trafficking of synaptotagmin via separate mechanisms. Synaptic vesicles immunoisolated from SV2A/B double knock-out mice had significantly less synaptotagmin than vesicles isolated from wild-type mice. Our results indicate that SV2 plays a major role in regulating the amount of synaptotagmin in synaptic vesicles and provide an explanation for the observation that synapses lacking SV2 have fewer vesicles competent for calcium-induced fusion.
Figures


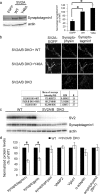
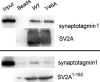

Similar articles
-
SV2 regulates neurotransmitter release via multiple mechanisms.Am J Physiol Cell Physiol. 2010 Nov;299(5):C960-7. doi: 10.1152/ajpcell.00259.2010. Epub 2010 Aug 11. Am J Physiol Cell Physiol. 2010. PMID: 20702688 Free PMC article.
-
Levetiracetam reverses synaptic deficits produced by overexpression of SV2A.PLoS One. 2011;6(12):e29560. doi: 10.1371/journal.pone.0029560. Epub 2011 Dec 29. PLoS One. 2011. PMID: 22220214 Free PMC article.
-
SV2A and SV2C contain a unique synaptotagmin-binding site.Mol Cell Neurosci. 2005 May;29(1):56-64. doi: 10.1016/j.mcn.2004.12.011. Mol Cell Neurosci. 2005. PMID: 15866046
-
Control of Synaptotagmin-1 Trafficking by SV2A-Mechanism and Consequences for Presynaptic Function and Dysfunction.J Neurochem. 2025 Jan;169(1):e16308. doi: 10.1111/jnc.16308. J Neurochem. 2025. PMID: 39853744 Free PMC article. Review.
-
Genetic and molecular analysis of the synaptotagmin family.Cell Mol Life Sci. 2001 Mar;58(3):393-402. doi: 10.1007/PL00000865. Cell Mol Life Sci. 2001. PMID: 11315187 Free PMC article. Review.
Cited by
-
Endocytic Adaptor Proteins in Health and Disease: Lessons from Model Organisms and Human Mutations.Cells. 2019 Oct 29;8(11):1345. doi: 10.3390/cells8111345. Cells. 2019. PMID: 31671891 Free PMC article. Review.
-
Connectivity Mapping Using a Novel sv2a Loss-of-Function Zebrafish Epilepsy Model as a Powerful Strategy for Anti-epileptic Drug Discovery.Front Mol Neurosci. 2022 May 24;15:881933. doi: 10.3389/fnmol.2022.881933. eCollection 2022. Front Mol Neurosci. 2022. PMID: 35686059 Free PMC article.
-
Molecular Signatures Underlying Synaptic Vesicle Cargo Retrieval.Front Cell Neurosci. 2018 Jan 5;11:422. doi: 10.3389/fncel.2017.00422. eCollection 2017. Front Cell Neurosci. 2018. PMID: 29379416 Free PMC article. Review.
-
Immunochemical analysis of the expression of SV2C in mouse, macaque and human brain.Brain Res. 2019 Jan 1;1702:85-95. doi: 10.1016/j.brainres.2017.12.029. Epub 2017 Dec 21. Brain Res. 2019. PMID: 29274878 Free PMC article.
-
Protein scaffolds in the coupling of synaptic exocytosis and endocytosis.Nat Rev Neurosci. 2011 Mar;12(3):127-38. doi: 10.1038/nrn2948. Epub 2011 Feb 9. Nat Rev Neurosci. 2011. PMID: 21304549 Review.
References
-
- Bushara KO, Malik T, Exconde RE. The effect of levetiracetam on essential tremor. Neurology. 2005;64:1078–1080. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
