Defective cAMP generation underlies the sensitivity of CNS neurons to neurofibromatosis-1 heterozygosity
- PMID: 20410111
- PMCID: PMC2864934
- DOI: 10.1523/JNEUROSCI.3994-09.2010
Defective cAMP generation underlies the sensitivity of CNS neurons to neurofibromatosis-1 heterozygosity
Abstract
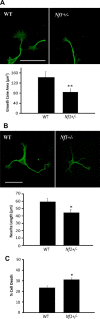
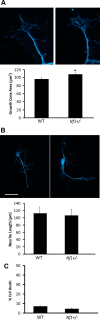
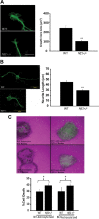
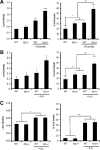
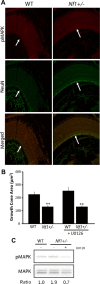
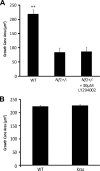
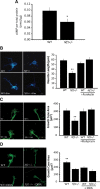
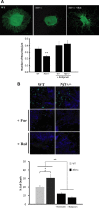
Individuals with the neurofibromatosis type 1 (NF1) inherited cancer syndrome exhibit neuronal dysfunction that predominantly affects the CNS. In this report, we demonstrate a unique vulnerability of CNS neurons, but not peripheral nervous system (PNS) neurons, to reduced Nf1 gene expression. Unlike dorsal root ganglion neurons, Nf1 heterozygous (Nf1+/-) hippocampal and retinal ganglion cell (RGC) neurons have decreased growth cone areas and neurite lengths, and increased apoptosis compared to their wild-type counterparts. These abnormal Nf1+/- CNS neuronal phenotypes do not reflect Ras pathway hyperactivation, but rather result from impaired neurofibromin-mediated cAMP generation. In this regard, elevating cAMP levels with forskolin or rolipram treatment, but not MEK (MAP kinase kinase) or PI3-K (phosphatidylinositol 3-kinase) inhibition, reverses these abnormalities to wild-type levels in vitro. In addition, Nf1+/- CNS, but not PNS, neurons exhibit increased apoptosis in response to excitotoxic or oxidative stress in vitro. Since children with NF1-associated optic gliomas often develop visual loss and Nf1 genetically engineered mice with optic glioma exhibit RGC neuronal apoptosis in vivo, we further demonstrate that RGC apoptosis resulting from optic glioma in Nf1 genetically engineered mice is attenuated by rolipram treatment in vivo. Similar to optic glioma-induced RGC apoptosis, the increased RGC neuronal death in Nf1+/- mice after optic nerve crush injury is also attenuated by rolipram treatment in vivo. Together, these findings establish a distinctive role for neurofibromin in CNS neurons with respect to vulnerability to injury, define a CNS-specific neurofibromin intracellular signaling pathway responsible for neuronal survival, and lay the foundation for future neuroprotective glioma treatment approaches.
Figures
Similar articles
-
Neurofibromatosis-1 heterozygosity impairs CNS neuronal morphology in a cAMP/PKA/ROCK-dependent manner.Mol Cell Neurosci. 2012 Jan;49(1):13-22. doi: 10.1016/j.mcn.2011.08.008. Epub 2011 Aug 26. Mol Cell Neurosci. 2012. PMID: 21903164 Free PMC article.
-
Akt- or MEK-mediated mTOR inhibition suppresses Nf1 optic glioma growth.Neuro Oncol. 2015 Jun;17(6):843-53. doi: 10.1093/neuonc/nou329. Epub 2014 Dec 21. Neuro Oncol. 2015. PMID: 25534823 Free PMC article.
-
Ultrastructural characterization of the optic pathway in a mouse model of neurofibromatosis-1 optic glioma.Neuroscience. 2010 Sep 29;170(1):178-88. doi: 10.1016/j.neuroscience.2010.06.017. Epub 2010 Jun 23. Neuroscience. 2010. PMID: 20600672 Free PMC article.
-
Insights into optic pathway glioma vision loss from mouse models of neurofibromatosis type 1.J Neurosci Res. 2019 Jan;97(1):45-56. doi: 10.1002/jnr.24250. Epub 2018 Apr 28. J Neurosci Res. 2019. PMID: 29704429 Free PMC article. Review.
-
Sensitization of Ion Channels Contributes to Central and Peripheral Dysfunction in Neurofibromatosis Type 1.Mol Neurobiol. 2017 Jul;54(5):3342-3349. doi: 10.1007/s12035-016-9907-1. Epub 2016 May 11. Mol Neurobiol. 2017. PMID: 27167129 Review.
Cited by
-
Associative Learning Requires Neurofibromin to Modulate GABAergic Inputs to Drosophila Mushroom Bodies.J Neurosci. 2021 Jun 16;41(24):5274-5286. doi: 10.1523/JNEUROSCI.1605-20.2021. Epub 2021 May 10. J Neurosci. 2021. PMID: 33972401 Free PMC article.
-
MOB2 suppresses GBM cell migration and invasion via regulation of FAK/Akt and cAMP/PKA signaling.Cell Death Dis. 2020 Apr 14;11(4):230. doi: 10.1038/s41419-020-2381-8. Cell Death Dis. 2020. PMID: 32286266 Free PMC article.
-
The therapeutic potential of neurofibromin signaling pathways and binding partners.Commun Biol. 2023 Apr 20;6(1):436. doi: 10.1038/s42003-023-04815-0. Commun Biol. 2023. PMID: 37081086 Free PMC article. Review.
-
Cyclic AMP is both a pro-apoptotic and anti-apoptotic second messenger.Acta Physiol (Oxf). 2012 Feb;204(2):277-87. doi: 10.1111/j.1748-1716.2011.02273.x. Epub 2011 May 26. Acta Physiol (Oxf). 2012. PMID: 21385327 Free PMC article. Review.
-
In vivo synaptic transmission and morphology in mouse models of Tuberous sclerosis, Fragile X syndrome, Neurofibromatosis type 1, and Costello syndrome.Front Cell Neurosci. 2015 Jul 3;9:234. doi: 10.3389/fncel.2015.00234. eCollection 2015. Front Cell Neurosci. 2015. PMID: 26190969 Free PMC article.
References
-
- Bajenaru ML, Hernandez MR, Perry A, Zhu Y, Parada LF, Garbow JR, Gutmann DH. Optic nerve glioma in mice requires astrocyte Nf1 gene inactivation and Nf1 brain heterozygosity. Cancer Res. 2003;63:8573–8577. - PubMed
-
- Barres BA, Silverstein BE, Corey DP, Chun LL. Immunological, morphological, and electrophysiological variation among retinal ganglion cells purified by panning. Neuron. 1988;1:791–803. - PubMed
-
- Basu TN, Gutmann DH, Fletcher JA, Glover TW, Collins FS, Downward J. Aberrant regulation of ras proteins in malignant tumour cells from type 1 neurofibromatosis patients. Nature. 1992;356:713–715. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
