Social interactions determine postural network sensitivity to 5-HT
- PMID: 20410113
- PMCID: PMC6632353
- DOI: 10.1523/JNEUROSCI.0367-10.2010
Social interactions determine postural network sensitivity to 5-HT
Abstract
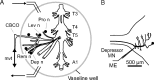
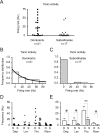
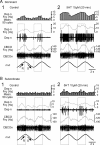
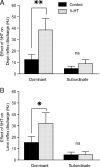
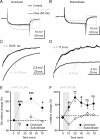
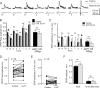
The excitability of the leg postural circuit and its response to serotonin (5-HT) were studied in vitro in thoracic nervous system preparations of dominant and subordinate male crayfishes. We demonstrate that the level of spontaneous tonic activity of depressor and levator motoneurons (MNs) (which control downward and upward movements of the leg, respectively) and the amplitude of their resistance reflex are larger in dominants than in subordinates. Moreover, we show that serotonergic neuromodulation of the postural circuit also depends on social status. Depressor and levator MN tonic firing rates and resistance reflex amplitudes were significantly modified in the presence of 10 mum 5-HT in dominants but not in subordinates. Using intracellular recording from depressor MNs, we show that their input resistance was not significantly different in dominants and subordinates in control conditions. However, 5-HT produced a marked depolarization in dominants and a significantly weaker depolarization in subordinates. Moreover, in the presence of 5-HT, the amplitude of the resistance reflex and the input resistance of MNs increased in dominants and decreased in subordinates. The peak amplitude and the decay phase of unitary EPSPs triggered by sensory spikes were significantly increased by 5-HT in dominants but not in subordinates. These observations suggest that neural networks are more reactive in dominants than in subordinates, and this divergence is even reinforced by 5-HT modulation.
Figures






Similar articles
-
Serotonin enhances the resistance reflex of the locomotor network of the crayfish through multiple modulatory effects that act cooperatively.J Neurosci. 2004 Jan 14;24(2):398-411. doi: 10.1523/JNEUROSCI.4032-03.2004. J Neurosci. 2004. PMID: 14724238 Free PMC article.
-
Inhibitory component of the resistance reflex in the locomotor network of the crayfish.J Neurophysiol. 2002 Nov;88(5):2575-88. doi: 10.1152/jn.00178.2002. J Neurophysiol. 2002. PMID: 12424295
-
Control of motor activity in crayfish by the steroid hormone 20-hydroxyecdysone via motoneuron excitability and sensory-motor integration.J Exp Biol. 2013 May 15;216(Pt 10):1808-18. doi: 10.1242/jeb.080176. Epub 2013 Feb 7. J Exp Biol. 2013. PMID: 23393273
-
Serotonin2c receptor constitutive activity: in vivo direct and indirect evidence and functional significance.Cent Nerv Syst Agents Med Chem. 2013 Jun;13(2):98-107. doi: 10.2174/1871524911313020002. Cent Nerv Syst Agents Med Chem. 2013. PMID: 23441866 Review.
-
Evolution of mirror systems: a simple mechanism for complex cognitive functions.Ann N Y Acad Sci. 2011 Apr;1225(1):166-75. doi: 10.1111/j.1749-6632.2011.06002.x. Ann N Y Acad Sci. 2011. PMID: 21535003 Free PMC article. Review.
Cited by
-
Aminergic control of social status in crayfish agonistic encounters.PLoS One. 2013 Sep 18;8(9):e74489. doi: 10.1371/journal.pone.0074489. eCollection 2013. PLoS One. 2013. PMID: 24058575 Free PMC article.
-
Neural circuit reconfiguration by social status.J Neurosci. 2012 Apr 18;32(16):5638-45. doi: 10.1523/JNEUROSCI.5668-11.2012. J Neurosci. 2012. PMID: 22514325 Free PMC article.
-
Enhancement of synaptic responses in ascending interneurones following acquisition of social dominance in crayfish.J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2021 May;207(3):415-428. doi: 10.1007/s00359-021-01481-7. Epub 2021 Mar 27. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2021. PMID: 33772639
-
The decision to fight or flee - insights into underlying mechanism in crickets.Front Neurosci. 2012 Aug 21;6:118. doi: 10.3389/fnins.2012.00118. eCollection 2012. Front Neurosci. 2012. PMID: 22936896 Free PMC article.
-
Serotonin precursor (5-hydroxytryptophan) causes substantial changes in the fighting behavior of male crickets, Gryllus bimaculatus.J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2013 Jul;199(7):601-9. doi: 10.1007/s00359-013-0804-z. Epub 2013 Mar 6. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2013. PMID: 23463029
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
