Nerve growth factor-regulated emergence of functional delta-opioid receptors
- PMID: 20410114
- PMCID: PMC2865237
- DOI: 10.1523/JNEUROSCI.5296-09.2010
Nerve growth factor-regulated emergence of functional delta-opioid receptors
Abstract
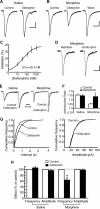
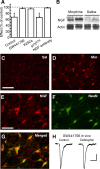
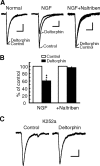
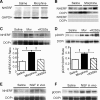
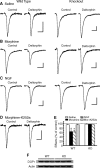
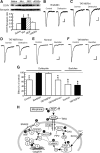
Sorting of intracellular G-protein-coupled receptors (GPCRs) either to lysosomes for degradation or to plasma membrane for surface insertion and functional expression is a key process regulating signaling strength of GPCRs across the plasma membrane in adult mammalian cells. However, little is known about the molecular mechanisms governing the dynamic process of receptor sorting to the plasma membrane for functional expression under normal and pathological conditions. In this study, we demonstrate that delta-opioid receptor (DOPr), a GPCR constitutively targeted to intracellular compartments, is driven to the surface membrane of central synaptic terminals and becomes functional by the neurotrophin nerve growth factor (NGF) in native brainstem neurons. The NGF-triggered DOPr translocation is predominantly mediated by the signaling pathway involving the tyrosine receptor kinase A, Ca(2+)-mobilizing phospholipase C, and Ca(2+)/calmodulin-dependent protein kinase II. Importantly, it requires interactions with the cytoplasmic sorting protein NHERF-1 (Na(+)/H(+) exchange regulatory factor-1) and N-ethyl-maleimide-sensitive factor-regulated exocytosis. In addition, this NGF-mediated mechanism is likely responsible for the emergence of functional DOPr induced by chronic opioids. Thus, NGF may function as a key molecular switch that redirects the sorting of intracellularly targeted DOPr to plasma membrane, resulting in new functional DOPr on central synapses under chronic opioid conditions.
Figures


Similar articles
-
Induction of delta-opioid receptor function in the midbrain after chronic morphine treatment.J Neurosci. 2005 Mar 23;25(12):3192-8. doi: 10.1523/JNEUROSCI.4585-04.2005. J Neurosci. 2005. PMID: 15788776 Free PMC article.
-
Neurotrophin-regulated sorting of opioid receptors in the biosynthetic pathway of neurosecretory cells.J Neurosci. 2003 Mar 15;23(6):2075-85. doi: 10.1523/JNEUROSCI.23-06-02075.2003. J Neurosci. 2003. PMID: 12657666 Free PMC article.
-
Synaptic mechanism for functional synergism between delta- and mu-opioid receptors.J Neurosci. 2010 Mar 31;30(13):4735-45. doi: 10.1523/JNEUROSCI.5968-09.2010. J Neurosci. 2010. PMID: 20357124 Free PMC article.
-
TRP Channel Trafficking.In: Liedtke WB, Heller S, editors. TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades. Boca Raton (FL): CRC Press/Taylor & Francis; 2007. Chapter 23. In: Liedtke WB, Heller S, editors. TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades. Boca Raton (FL): CRC Press/Taylor & Francis; 2007. Chapter 23. PMID: 21204515 Free Books & Documents. Review.
-
Aiming at Ideal Therapeutics-MOPr/DOPr or MOPr-DOPr Heteromertargeting Ligand.Curr Top Med Chem. 2020;20(31):2843-2851. doi: 10.2174/1568026620666200423095231. Curr Top Med Chem. 2020. PMID: 32324516 Review.
Cited by
-
NGF, BDNF and Arc mRNA Expression in the Hippocampus of Rats After Administration of Morphine.Neurochem Res. 2019 Sep;44(9):2139-2146. doi: 10.1007/s11064-019-02851-z. Epub 2019 Aug 2. Neurochem Res. 2019. PMID: 31376054
-
Nerve growth factor sensitizes adult sympathetic neurons to the proinflammatory peptide bradykinin.J Neurosci. 2014 Sep 3;34(36):11959-71. doi: 10.1523/JNEUROSCI.1536-14.2014. J Neurosci. 2014. PMID: 25186743 Free PMC article.
-
Emergence of functional spinal delta opioid receptors after chronic ethanol exposure.Biol Psychiatry. 2012 Feb 1;71(3):232-8. doi: 10.1016/j.biopsych.2011.07.015. Epub 2011 Sep 1. Biol Psychiatry. 2012. PMID: 21889123 Free PMC article.
-
Pharmacological traits of delta opioid receptors: pitfalls or opportunities?Psychopharmacology (Berl). 2013 Jul;228(1):1-18. doi: 10.1007/s00213-013-3129-2. Epub 2013 May 7. Psychopharmacology (Berl). 2013. PMID: 23649885 Free PMC article. Review.
-
Sex Differences in the Rat Hippocampal Opioid System After Oxycodone Conditioned Place Preference.Neuroscience. 2018 Nov 21;393:236-257. doi: 10.1016/j.neuroscience.2018.10.002. Epub 2018 Oct 11. Neuroscience. 2018. PMID: 30316908 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
