Insulin-regulated aminopeptidase is a key regulator of GLUT4 trafficking by controlling the sorting of GLUT4 from endosomes to specialized insulin-regulated vesicles
- PMID: 20410133
- PMCID: PMC2883947
- DOI: 10.1091/mbc.e10-02-0158
Insulin-regulated aminopeptidase is a key regulator of GLUT4 trafficking by controlling the sorting of GLUT4 from endosomes to specialized insulin-regulated vesicles
Abstract
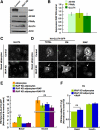
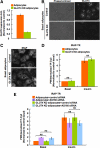
Insulin stimulates glucose uptake by regulating translocation of the GLUT4 glucose transporter from intracellular compartments to the plasma membrane. In the absence of insulin GLUT4 is actively sequestered away from the general endosomes into GLUT4-specialized compartments, thereby controlling the amount of GLUT4 at the plasma membrane. Here, we investigated the role of the aminopeptidase IRAP in GLUT4 trafficking. In unstimulated IRAP knockdown adipocytes, plasma membrane GLUT4 levels are elevated because of increased exocytosis, demonstrating an essential role of IRAP in GLUT4 retention. Current evidence supports the model that AS160 RabGAP, which is required for basal GLUT4 retention, is recruited to GLUT4 compartments via an interaction with IRAP. However, here we show that AS160 recruitment to GLUT4 compartments and AS160 regulation of GLUT4 trafficking were unaffected by IRAP knockdown. These results demonstrate that AS160 is recruited to membranes by an IRAP-independent mechanism. Consistent with a role independent of AS160, we showed that IRAP functions in GLUT4 sorting from endosomes to GLUT4-specialized compartments. This is revealed by the relocalization of GLUT4 to endosomes in IRAP knockdown cells. Although IRAP knockdown has profound effects on GLUT4 traffic, GLUT4 knockdown does not affect IRAP trafficking, demonstrating that IRAP traffics independent of GLUT4. In sum, we show that IRAP is both cargo and a key regulator of the insulin-regulated pathway.
Figures



References
-
- Abel E. D., Graveleau C., Betuing S., Pham M., Reay P. A., Kandror V., Kupriyanova T., Xu Z., Kandror K. V. Regulation of insulin-responsive aminopeptidase expression and targeting in the insulin-responsive vesicle compartment of glucose transporter isoform 4-deficient cardiomyocytes. Mol. Endocrinol. 2004;18:2491–2501. - PubMed
-
- Albiston A. L., et al. Evidence that the angiotensin IV (AT(4)) receptor is the enzyme insulin-regulated aminopeptidase. J. Biol. Chem. 2001;276:48623–48626. - PubMed
-
- Albiston A. L., Mustafa T., McDowall S. G., Mendelsohn F. A., Lee J., Chai S. Y. AT4 receptor is insulin-regulated membrane aminopeptidase: potential mechanisms of memory enhancement. Trends Endocrinol. Metab. 2003;14:72–77. - PubMed
-
- Albiston A. L., Peck G. R., Yeatman H. R., Fernando R., Ye S., Chai S. Y. Therapeutic targeting of insulin-regulated aminopeptidase: heads and tails? Pharmacol. Ther. 2007;116:417–427. - PubMed
-
- Antonescu C. N., Foti M., Sauvonnet N., Klip A. Ready, set, internalize: mechanisms and regulation of GLUT4 endocytosis. Biosci. Rep. 2009;29:1–11. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

