O-GlcNAc cycling enzymes associate with the translational machinery and modify core ribosomal proteins
- PMID: 20410138
- PMCID: PMC2883937
- DOI: 10.1091/mbc.e09-11-0941
O-GlcNAc cycling enzymes associate with the translational machinery and modify core ribosomal proteins
Abstract
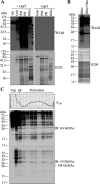
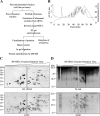
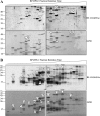
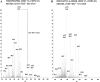
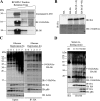
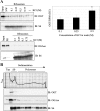
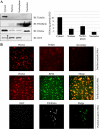
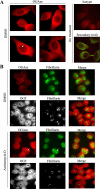
Protein synthesis is globally regulated through posttranslational modifications of initiation and elongation factors. Recent high-throughput studies have identified translation factors and ribosomal proteins (RPs) as substrates for the O-GlcNAc modification. Here we determine the extent and abundance of O-GlcNAcylated proteins in translational preparations. O-GlcNAc is present on many proteins that form active polysomes. We identify twenty O-GlcNAcylated core RPs, of which eight are newly reported. We map sites of O-GlcNAc modification on four RPs (L6, L29, L32, and L36). RPS6, a component of the mammalian target of rapamycin (mTOR) signaling pathway, follows different dynamics of O-GlcNAcylation than nutrient-induced phosphorylation. We also show that both O-GlcNAc cycling enzymes OGT and OGAse strongly associate with cytosolic ribosomes. Immunofluorescence experiments demonstrate that OGAse is present uniformly throughout the nucleus, whereas OGT is excluded from the nucleolus. Moreover, nucleolar stress only alters OGAse nuclear staining, but not OGT staining. Lastly, adenovirus-mediated overexpression of OGT, but not of OGAse or GFP control, causes an accumulation of 60S subunits and 80S monosomes. Our results not only establish that O-GlcNAcylation extensively modifies RPs, but also suggest that O-GlcNAc play important roles in regulating translation and ribosome biogenesis.
Figures

Similar articles
-
O-GlcNAcylation of ribosome-associated proteins is concomitant with translational reprogramming during proteotoxic stress.J Biol Chem. 2024 Nov;300(11):107877. doi: 10.1016/j.jbc.2024.107877. Epub 2024 Oct 10. J Biol Chem. 2024. PMID: 39395807 Free PMC article.
-
O-linked beta-N-acetylglucosamine (O-GlcNAc): Extensive crosstalk with phosphorylation to regulate signaling and transcription in response to nutrients and stress.Biochim Biophys Acta. 2010 Feb;1800(2):96-106. doi: 10.1016/j.bbagen.2009.07.018. Epub 2009 Aug 6. Biochim Biophys Acta. 2010. PMID: 19647786 Free PMC article. Review.
-
Cross-talk between two essential nutrient-sensitive enzymes: O-GlcNAc transferase (OGT) and AMP-activated protein kinase (AMPK).J Biol Chem. 2014 Apr 11;289(15):10592-10606. doi: 10.1074/jbc.M113.523068. Epub 2014 Feb 21. J Biol Chem. 2014. PMID: 24563466 Free PMC article.
-
Feedback Regulation of O-GlcNAc Transferase through Translation Control to Maintain Intracellular O-GlcNAc Homeostasis.Int J Mol Sci. 2021 Mar 27;22(7):3463. doi: 10.3390/ijms22073463. Int J Mol Sci. 2021. PMID: 33801653 Free PMC article.
-
Intracellular protein O-GlcNAc modification integrates nutrient status with transcriptional and metabolic regulation.Adv Cancer Res. 2015;126:137-66. doi: 10.1016/bs.acr.2014.12.003. Epub 2015 Feb 7. Adv Cancer Res. 2015. PMID: 25727147 Review.
Cited by
-
A missense mutation in the catalytic domain of O-GlcNAc transferase links perturbations in protein O-GlcNAcylation to X-linked intellectual disability.FEBS Lett. 2020 Feb;594(4):717-727. doi: 10.1002/1873-3468.13640. Epub 2019 Nov 7. FEBS Lett. 2020. PMID: 31627256 Free PMC article.
-
Cryo-EM structure provides insights into the dimer arrangement of the O-linked β-N-acetylglucosamine transferase OGT.Nat Commun. 2021 Nov 11;12(1):6508. doi: 10.1038/s41467-021-26796-6. Nat Commun. 2021. PMID: 34764280 Free PMC article.
-
Hyperglycemia mediates a shift from cap-dependent to cap-independent translation via a 4E-BP1-dependent mechanism.Diabetes. 2013 Jul;62(7):2204-14. doi: 10.2337/db12-1453. Epub 2013 Feb 22. Diabetes. 2013. PMID: 23434932 Free PMC article.
-
Invariable stoichiometry of ribosomal proteins in mouse brain tissues with aging.Proc Natl Acad Sci U S A. 2019 Nov 5;116(45):22567-22572. doi: 10.1073/pnas.1912060116. Epub 2019 Oct 21. Proc Natl Acad Sci U S A. 2019. PMID: 31636180 Free PMC article.
-
O-GlcNAcylation mediates H2O2-induced apoptosis through regulation of STAT3 and FOXO1.Acta Pharmacol Sin. 2024 Apr;45(4):714-727. doi: 10.1038/s41401-023-01218-z. Epub 2024 Jan 8. Acta Pharmacol Sin. 2024. PMID: 38191912 Free PMC article.
References
-
- Acker M. G., Lorsch J. R. Mechanism of ribosomal subunit joining during eukaryotic translation initiation. Biochem. Soc. Trans. 2008;36:653–657. - PubMed
-
- Akimoto Y., Kreppel L. K., Hirano H., Hart G. W. Localization of the O-linked N-acetylglucosamine transferase in rat pancreas. Diabetes. 1999;48:2407–2413. - PubMed
-
- Andersen J. S., Lam Y. W., Leung A. K., Ong S. E., Lyon C. E., Lamond A. I., Mann M. Nucleolar proteome dynamics. Nature. 2005;433:77–83. - PubMed
-
- Ban N., Nissen P., Hansen J., Moore P. B., Steitz T. A. The complete atomic structure of the large ribosomal subunit at 2.4 A resolution. Science. 2000;289:905–920. - PubMed
-
- Boisvert F. M., van Koningsbruggen S., Navascues J., Lamond A. I. The multifunctional nucleolus. Nat. Rev. Mol. Cell Biol. 2007;8:574–585. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

