Neuralized promotes basal to apical transcytosis of delta in epithelial cells
- PMID: 20410139
- PMCID: PMC2883951
- DOI: 10.1091/mbc.e09-11-0926
Neuralized promotes basal to apical transcytosis of delta in epithelial cells
Abstract
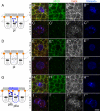
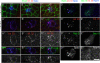
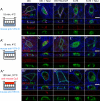
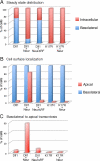
Notch receptors mediate short-range signaling controlling many developmental decisions in metazoans. Activation of Notch requires the ubiquitin-dependent endocytosis of its ligand Delta. How ligand endocytosis in signal-sending cells regulates receptor activation in juxtaposed signal-receiving cells remains largely unknown. We show here that a pool of Delta localizes at the basolateral membrane of signal-sending sensory organ precursor cells in the dorsal thorax neuroepithelium of Drosophila and that Delta is endocytosed in a Neuralized-dependent manner from this basolateral membrane. This basolateral pool of Delta is segregated from Notch that accumulates apically. Using a compartimentalized antibody uptake assay, we show that murine Delta-like 1 is similarly internalized by mNeuralized2 from the basolateral membrane of polarized Madin-Darby canine kidney cells and that internalized ligands are transcytosed to the apical plasma membrane where mNotch1 accumulates. Thus, endocytosis of Delta by Neuralized relocalizes Delta from the basolateral to the apical membrane domain. We speculate that this Neuralized-dependent transcytosis regulates the signaling activity of Delta by relocalizing Delta from a membrane domain where it cannot interact with Notch to another membrane domain where it can bind and activate Notch.
Figures


References
-
- Bardin A. J., Schweisguth F. Bearded family members inhibit Neuralized-mediated endocytosis and signaling activity of Delta in Drosophila. Dev. Cell. 2006;10:245–255. - PubMed
-
- Carraway C. A., Carraway K. L. Sequestration and segregation of receptor kinases in epithelial cells: implications for ErbB2 oncogenesis. Sci. STKE. 2007;2007:re3. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

