TRIM5alpha disrupts the structure of assembled HIV-1 capsid complexes in vitro
- PMID: 20410272
- PMCID: PMC2903270
- DOI: 10.1128/JVI.00210-10
TRIM5alpha disrupts the structure of assembled HIV-1 capsid complexes in vitro
Abstract
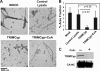
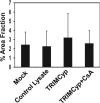
The host restriction factor TRIM5alpha provides intrinsic defense against retroviral infections in mammalian cells. TRIM5alpha blocks infection by targeting the viral capsid after entry but prior to completion of reverse transcription, but whether this interaction directly alters the structure of the viral capsid is unknown. A previous study reported that rhesus macaque TRIM5alpha protein stably associates with cylindrical complexes formed by assembly of recombinant HIV-1 CA-NC protein in vitro and that restriction leads to accelerated HIV-1 uncoating in target cells. To gain further insight into the mechanism of TRIM5alpha-dependent restriction, we examined the structural effects of TRIM5 proteins on preassembled CA-NC complexes by electron microscopy. Incubation of assembled complexes with lysate of cells expressing the restrictive rhesus TRIM5alpha protein resulted in marked disruption of the normal cylindrical structure of the complexes. In contrast, incubation with lysate of control cells or cells expressing comparable levels of the nonrestrictive human TRIM5alpha protein had little effect on the complexes. Incubation with lysate of cells expressing the TRIMCyp restriction factor also disrupted the cylinders. The effect of TRIMCyp was prevented by the addition of cyclosporine, which inhibits binding of TRIMCyp to the HIV-1 capsid. Thus, disruption of CA-NC cylinders by TRIM5alpha and TRIMCyp was correlated with the specificity of restriction. Collectively, these results suggest that TRIM5alpha-dependent restriction of HIV-1 infection results from structural perturbation of the viral capsid leading to aberrant HIV-1 uncoating in target cells.
Figures

References
-
- Anderson, J., and R. Akkina. 2005. TRIM5alpharh expression restricts HIV-1 infection in lentiviral vector-transduced CD34+-cell-derived macrophages. Mol. Ther. 12:687-696. - PubMed
-
- Bieniasz, P. D. 2004. Intrinsic immunity: a front-line defense against viral attack. Nat. Immunol. 5:1109-1115. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

