A combination of biochemical and proteomic analyses reveals Bx-LEC-1 as an antigenic target for the monoclonal antibody 3-2A7-2H5-D9-F10 specific to the pine wood nematode
- PMID: 20410377
- PMCID: PMC3033686
- DOI: 10.1074/mcp.M900521-MCP200
A combination of biochemical and proteomic analyses reveals Bx-LEC-1 as an antigenic target for the monoclonal antibody 3-2A7-2H5-D9-F10 specific to the pine wood nematode
Abstract
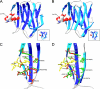
Pine wilt disease (PWD) is one of the most devastating forest diseases in Asia and Europe. The pine wood nematode, Bursaphelenchus xylophilus, has been identified as the pathogen underlying PWD, although the pathology is not completely understood. At present, diagnosis and confirmation of PWD are time consuming tasks that require nematode extraction and microscopic examination. To develop a more efficient detection method for B. xylophilus, we first generated monoclonal antibodies (MAbs) specific to B. xylophilus. Among 2304 hybridoma fusions screened, a hybridoma clone named 3-2A7-2H5 recognized a single protein from B. xylophilus specifically, but not those from other closely related nematodes. We finally selected the MAb clone 3-2A7-2H5-D9-F10 (D9-F10) for further studies. To identify the antigenic target of MAb-D9-F10, we analyzed proteins in spots, fractions, or bands isolated from SDS-PAGE, two-dimensional electrophoresis, anion exchange chromatography, and immunoprecipitation via nano liquid chromatography electrospray ionization quadrupole ion trap mass spectrometry (nano-LC-ESI-Q-IT-MS). Peptides of galactose-binding lectin-1 of B. xylophilus (Bx-LEC-1) were commonly detected in several proteomic analyses, demonstrating that this LEC-1 is the antigenic target of MAb-D9-F10. The localization of MAb-D9-F10 immunoreactivities at the area of the median bulb and esophageal glands suggested that the Bx-LEC-1 may be involved in food perception and digestion. The Bx-LEC-1 has two nonidentical galactose-binding lectin domains important for carbohydrate binding. The affinity of the Bx-LEC-1 to D-(+)-raffinose and N-acetyllactosamine were much higher than that to L-(+)-rhamnose. Based on this combination of evidences, MAb-D9-F10 is the first identified molecular biomarker specific to the Bx-LEC-1.
Figures






References
-
- Steiner G., Buhrer E. M. (1934) Aphelenchoides xylophilus, n. sp., a nematode associated with blue-stain and other fungi in timber. J. Agric. Res. 48, 949–951
-
- Yano S. (1913) Investigation on pine death in Nagasaki prefecture. Sanrin-Kouhou 4, 1–14
-
- Kiyohara T., Tokushige Y. (1971) Inoculation experiments of a nematode, Bursaphelenchus sp., onto pine trees. J. Jpn. For. Soc. 53, 210–218
-
- Enda N. (1989) Current status of pine wilt disease in Korea. Forest Pests 38, 148–152
-
- Yang B., Wang Q. (1989) Distribution of the pine wood nematode in China and susceptibility of some Chinese and exotic pine to the nematode. Can. J. For. Res. 19, 1527–1530
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

