Molecular mechanisms of amphetamine actions in Caenorhabditis elegans
- PMID: 20410438
- PMCID: PMC2912056
- DOI: 10.1124/mol.109.062703
Molecular mechanisms of amphetamine actions in Caenorhabditis elegans
Abstract
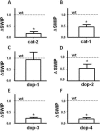
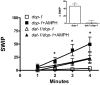
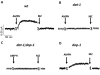
Amphetamine (AMPH) poses a serious hazard to public health. Defining the molecular targets of AMPH is essential to developing treatments for psychostimulant abuse. AMPH elicits its behavioral effects primarily by increasing extracellular dopamine (DA) levels through the reversal of the DA transporter (DAT) cycle and, as a consequence, altering DA signaling. In Caenorhabditis elegans, an excess of synaptic DA results in a loss of motility in water, termed swimming-induced paralysis (SWIP). Here we demonstrate that AMPH produces SWIP in a time- and dose-dependent manner in wild-type (wt) animals but has a reduced ability to generate SWIP in DAT knock out worms (dat-1). To determine whether D1-like and/or D2-like receptors are involved in AMPH-induced SWIP, we performed experiments in DOP-1 and DOP-4, and DOP-2, and DOP-3 receptor knockout animals, respectively. AMPH administration resulted in a reduced ability to induce SWIP in animals lacking DOP-3, DOP-4, and DOP-2 receptors. In contrast, in worms lacking DOP-1 receptors, AMPH-induced SWIP occurred at wt levels. Using microamperometry on C. elegans DA neurons, we determined that in contrast to wt cells, AMPH failed to promote DA efflux in dat-1 DA neurons. These data suggest that DA efflux is critical to sustaining SWIP behavior by signaling through DOP-3, DOP-4, and DOP-2. In a double mutant lacking both DAT-1 and DOP-1 expression, we found no ability of AMPH to induce SWIP or DA efflux. This result supports the paradigm that DA efflux through C. elegans DAT is required for AMPH-induced behaviors and does not require DOP-1 signaling.
Figures

References
-
- Ball KT, Budreau D, Rebec GV. (2003) Acute effects of 3,4-methylenedioxymethamphetamine on striatal single-unit activity and behavior in freely moving rats: differential involvement of dopamine D(1) and D(2) receptors. Brain Res 994:203–215 - PubMed
-
- Barstead RJ, Kleiman L, Waterston RH. (1991) Cloning, sequencing, and mapping of an alpha-actinin gene from the nematode Caenorhabditis elegans. Cell Motil Cytoskeleton 20:69–78 - PubMed
-
- Bettinger JC, McIntire SL. (2004) State-dependency in C. elegans. Genes Brain Behav 3:266–272 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

