Efficient generation of lens progenitor cells and lentoid bodies from human embryonic stem cells in chemically defined conditions
- PMID: 20410439
- PMCID: PMC2923359
- DOI: 10.1096/fj.10-157255
Efficient generation of lens progenitor cells and lentoid bodies from human embryonic stem cells in chemically defined conditions
Abstract
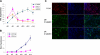
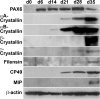
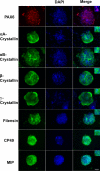
The eye lens is an encapsulated avascular organ whose function is to focus light on the retina. Lens comprises a single progenitor cell lineage in multiple states of differentiation. Disruption of lens function leading to protein aggregation and opacity results in age-onset cataract. Cataract is a complex disease involving genetic and environmental factors. Here, we report the development of a new 3-stage system that differentiates human embryonic stem cells (hESCs) into large quantities of lens progenitor-like cells and differentiated 3-dimensional lentoid bodies. Inhibition of BMP signaling by noggin triggered differentiation of hESCs toward neuroectoderm. Subsequent reactivation of BMP and activation of FGF signaling stimulated formation of lens progenitor cells marked by the expression of PAX6 and alpha-crystallins. The formation of lentoid bodies was most efficient in the presence of FGF2 and Wnt-3a, yielding approximately 1000 lentoid bodies/30-mm well. Lentoid bodies expressed and accumulated lens-specific markers including alphaA-, alphaB-, beta-, and gamma-crystallins, filensin, CP49, and MIP/aquaporin 0. Collectively, these studies identify a novel procedure to generate lens cells from hESCs that can be applied for studies of lens differentiation and cataractogenesis using induced pluripotent stem (iPS) cells derived from various cataract patients.
Figures




Similar articles
-
Generation of Lens Progenitor Cells and Lentoid Bodies from Pluripotent Stem Cells: Novel Tools for Human Lens Development and Ocular Disease Etiology.Cells. 2022 Nov 6;11(21):3516. doi: 10.3390/cells11213516. Cells. 2022. PMID: 36359912 Free PMC article. Review.
-
Wnt5a Contributes to the Differentiation of Human Embryonic Stem Cells into Lentoid Bodies Through the Noncanonical Wnt/JNK Signaling Pathway.Invest Ophthalmol Vis Sci. 2018 Jul 2;59(8):3449-3460. doi: 10.1167/iovs.18-23902. Invest Ophthalmol Vis Sci. 2018. PMID: 30025083
-
Generation of Functional Lentoid Bodies From Human Induced Pluripotent Stem Cells Derived From Urinary Cells.Invest Ophthalmol Vis Sci. 2017 Jan 1;58(1):517-527. doi: 10.1167/iovs.16-20504. Invest Ophthalmol Vis Sci. 2017. PMID: 28125839
-
Efficient generation of lens progenitor cells from cataract patient-specific induced pluripotent stem cells.PLoS One. 2012;7(3):e32612. doi: 10.1371/journal.pone.0032612. Epub 2012 Mar 5. PLoS One. 2012. PMID: 22403680 Free PMC article.
-
Lens regeneration: scientific discoveries and clinical possibilities.Mol Biol Rep. 2021 May;48(5):4911-4923. doi: 10.1007/s11033-021-06489-5. Epub 2021 Jun 18. Mol Biol Rep. 2021. PMID: 34143397 Review.
Cited by
-
Molecular characterization of mouse lens epithelial cell lines and their suitability to study RNA granules and cataract associated genes.Exp Eye Res. 2015 Feb;131:42-55. doi: 10.1016/j.exer.2014.12.011. Epub 2014 Dec 19. Exp Eye Res. 2015. PMID: 25530357 Free PMC article.
-
Generation of Lens Progenitor Cells and Lentoid Bodies from Pluripotent Stem Cells: Novel Tools for Human Lens Development and Ocular Disease Etiology.Cells. 2022 Nov 6;11(21):3516. doi: 10.3390/cells11213516. Cells. 2022. PMID: 36359912 Free PMC article. Review.
-
In vitro modeling of cranial placode differentiation: Recent advances, challenges, and perspectives.Dev Biol. 2024 Feb;506:20-30. doi: 10.1016/j.ydbio.2023.11.009. Epub 2023 Dec 3. Dev Biol. 2024. PMID: 38052294 Free PMC article. Review.
-
Reversible cold-induced lens opacity in a hibernator reveals a molecular target for treating cataracts.J Clin Invest. 2024 Sep 17;134(18):e169666. doi: 10.1172/JCI169666. J Clin Invest. 2024. PMID: 39286982 Free PMC article.
-
Pigment Epithelia of the Eye: Cell-Type Conversion in Regeneration and Disease.Life (Basel). 2022 Mar 6;12(3):382. doi: 10.3390/life12030382. Life (Basel). 2022. PMID: 35330132 Free PMC article. Review.
References
-
- Streit A. Early development of the cranial sensory nervous system: from a common field to individual placodes. Dev Biol. 2004;276:1–15. - PubMed
-
- Lang R A. Pathways regulating lens induction in the mouse. Int J Dev Biol. 2004;48:783–791. - PubMed
-
- Lovicu F J, McAvoy J W. Growth factor regulation of lens development. Dev Biol. 2005;280:1–14. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

