Hematopoietic and nonhematopoietic cell tissue factor activates the coagulation cascade in endotoxemic mice
- PMID: 20410508
- PMCID: PMC2918334
- DOI: 10.1182/blood-2009-12-259267
Hematopoietic and nonhematopoietic cell tissue factor activates the coagulation cascade in endotoxemic mice
Abstract
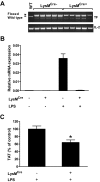
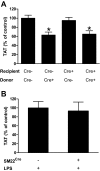
Tissue factor (TF) is the primary activator of the coagulation cascade. During endotoxemia, TF expression leads to disseminated intravascular coagulation. However, the relative contribution of TF expression by different cell types to the activation of coagulation has not been defined. In this study, we investigated the effect of either a selective inhibition of TF expression or cell type-specific deletion of the TF gene (F3) on activation of coagulation in a mouse model of endotoxemia. We found that inhibition of TF on either hematopoietic or nonhematopoietic cells reduced plasma thrombin-antithrombin (TAT) levels 8 hours after administration of bacterial lipopolysaccharide (LPS). In addition, plasma TAT levels were significantly reduced in endotoxemic mice lacking the TF gene in either myeloid cells (TF(flox/flox),LysM(Cre) mice) or in both endothelial cells (ECs) and hematopoietic cells (TF(flox/flox),Tie-2(Cre) mice). However, deletion of the TF gene in ECs alone had no effect on LPS-induced plasma TAT levels. Similar results were observed in mice lacking TF in vascular smooth muscle cells. Finally, we found that mouse platelets do not express TF pre-mRNA or mRNA. Our data demonstrate that in a mouse model of endotoxemia activation of the coagulation cascade is initiated by TF expressed by myeloid cells and an unidentified nonhematopoietic cell type(s).
Figures
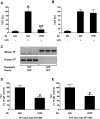


 indicates ECs. Incubation of serial sections with control rat IgG demonstrated no staining (data not shown).
indicates ECs. Incubation of serial sections with control rat IgG demonstrated no staining (data not shown).
Comment in
-
O tissue factor, where art thou?Blood. 2010 Aug 5;116(5):676-7. doi: 10.1182/blood-2010-05-283135. Blood. 2010. PMID: 20688964 No abstract available.
References
-
- Levi M. Disseminated intravascular coagulation: What's new? Crit Care Clin. 2005;21(3):449–467. - PubMed
-
- Taylor FB, Jr, Chang A, Ruf W, et al. Lethal E. coli septic shock is prevented by blocking tissue factor with monoclonal antibody. Circ Shock. 1991;33(3):127–134. - PubMed
-
- Taylor FB, Chang AC, Peer G, Li A, Ezban M, Hedner U. Active site inhibited factor VIIa (DEGR VIIa) attenuates the coagulant and interleukin-6 and -8, but not tumor necrosis factor, responses of the baboon to LD100 Escherichia coli. Blood. 1998;91(5):1609–1615. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

