Development of a nonintegrating Rev-dependent lentiviral vector carrying diphtheria toxin A chain and human TRAF6 to target HIV reservoirs
- PMID: 20410930
- PMCID: PMC2910233
- DOI: 10.1038/gt.2010.53
Development of a nonintegrating Rev-dependent lentiviral vector carrying diphtheria toxin A chain and human TRAF6 to target HIV reservoirs
Abstract
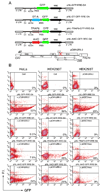
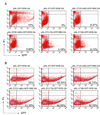
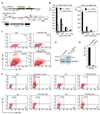
Persistence of human immunodeficiency virus (HIV) despite highly active antiretroviral therapy (HAART) is a lasting challenge to virus eradication. To develop a strategy complementary to HAART, we constructed a series of Rev-dependent lentiviral vectors carrying diphtheria toxin A chain (DT-A) and its attenuated mutants, as well as human tumor necrosis factor receptor-associated factor 6 (TRAF6). Expression of these suicide genes following delivery through viral particles is dependent on Rev, which exists only in infected cells. Among these toxins, DT-A has been known to trigger cell death with as little as a single molecule, whereas two of the attenuated mutants in this study, DT-A(176) and DT-A(Delta N), were well tolerated by cells at low levels. TRAF6 induced apoptosis only with persistent overexpression. Thus, these suicide genes, which induce cell death at different expression levels, offer a balance between efficacy and safety. To minimize possible mutagenesis introduced by retroviral integration in nontarget cells, we further developed a nonintegrating Rev-dependent (NIRD) lentiviral vector to deliver these genes. In addition, we constructed a DT-A-resistant human cell line by introducing a human elongation factor 2 mutant into HEK293T cells. This allowed us to manufacture the first high-titer NIRD lentiviral particles carrying DT-A to target HIV-positive cells.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Torres RA, Barr M. Impact of combination therapy for HIV infection on inpatient census. N Engl J Med. 1997;336:1531–1532. - PubMed
-
- Ramratnam B, Mittler JE, Zhang L, Boden D, Hurley A, Fang F, et al. The decay of the latent reservoir of replication-competent HIV-1 is inversely correlated with the extent of residual viral replication during prolonged anti-retroviral therapy. Nat Med. 2000;6:82–85. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

