Role of inflammation in túbulo-interstitial damage associated to obstructive nephropathy
- PMID: 20412564
- PMCID: PMC2873503
- DOI: 10.1186/1476-9255-7-19
Role of inflammation in túbulo-interstitial damage associated to obstructive nephropathy
Abstract
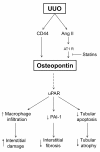
Obstructive nephropathy is characterized by an inflammatory state in the kidney, that is promoted by cytokines and growth factors produced by damaged tubular cells, infiltrated macrophages and accumulated myofibroblasts. This inflammatory state contributes to tubular atrophy and interstitial fibrosis characteristic of obstructive nephropathy. Accumulation of leukocytes, especially macrophages and T lymphocytes, in the renal interstitium is strongly associated to the progression of renal injury. Proinflammatory cytokines, NF-kappaB activation, adhesion molecules, chemokines, growth factors, NO and oxidative stress contribute in different ways to progressive renal damage induced by obstructive nephropathy, as they induce leukocytes recruitment, tubular cell apoptosis and interstitial fibrosis. Increased angiotensin II production, increased oxidative stress and high levels of proinflammatory cytokines contribute to NF-kappaB activation which in turn induce the expression of adhesion molecules and chemokines responsible for leukocyte recruitment and iNOS and cytokines overexpression, which aggravates the inflammatory response in the damaged kidney. In this manuscript we revise the different events and regulatory mechanisms involved in inflammation associated to obstructive nephropathy.
Figures
References
-
- Klahr S, Morrissey J. Obstructive nephropathy and renal fibrosis. Am J Physiol Renal Physiol. 2002;283:F861–F875. - PubMed
LinkOut - more resources
Full Text Sources

