Spontaneous feline mammary intraepithelial lesions as a model for human estrogen receptor- and progesterone receptor-negative breast lesions
- PMID: 20412586
- PMCID: PMC2873946
- DOI: 10.1186/1471-2407-10-156
Spontaneous feline mammary intraepithelial lesions as a model for human estrogen receptor- and progesterone receptor-negative breast lesions
Abstract
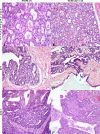
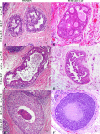
Background: Breast cancer is the most frequently diagnosed cancer in women. Intraepithelial lesions (IELs), such as usual ductal hyperplasia (UH), atypical ductal hyperplasia (ADH), and ductal carcinoma in situ (DCIS) are risk factors that predict a woman's chance of developing invasive breast cancer. Therefore, a comparative study that establishes an animal model of pre-invasive lesions is needed for the development of preventative measures and effective treatment for both mammary IELs and tumors. The purpose of this study was to characterize the histologic and molecular features of feline mammary IELs and compare them with those in women.
Methods: Formalin-fixed, paraffin-embedded specimens (n = 205) from 203 female cats with clinical mammary disease were retrieved from the archives of the Purdue University Animal Disease Diagnostic Laboratory and Veterinary Teaching Hospital (West Lafayette, IN), and the Department of Pathology and Veterinary Clinic, School of Veterinary Medicine (Sassari, Italy). Histologic sections, stained with hematoxylin and eosin (HE), were evaluated for the presence of IELs in tissue adjacent to excised mammary tumors. Lesions were compared to those of humans. Immunohistochemistry for estrogen receptor (ER-alpha), progesterone receptor (PR), human epidermal growth factor receptor 2 (HER-2/neu) and Ki-67 was performed in IELs and adjacent tumor tissues.
Results: Intraepithelial lesions were found in 57 of 203 (28%) feline mammary specimens and were categorized as UH (27%), ADH (29%), and DCIS (44%). Most IELs with atypia (ADH and DCIS) were associated with mammary cancer (91%), whereas UH was associated with benign lesions in 53% of cases. Feline IELs were remarkably similar to human IELs. No ER or PR immunoreactivity was detected in intermediate-grade or high-grade DCIS or their associated malignant tumors. HER-2 protein overexpression was found in 27% of IELs.
Conclusion: The remarkable similarity of feline mammary IELs to those of humans, with the tendency to lose hormone receptor expression in atypical IELs, supports the cat as a possible model to study ER- and PR-negative breast lesions.
Figures

References
-
- O'Shaughnessy JA, Kelloff GJ, Gordon GB, Dannenberg AJ, Hong WK, Fabian CJ, Sigman CC, Bertagnolli MM, Stratton SP, Lam S, Nelson WG, Meyskens FL, Alberts DS, Follen M, Rustgi AK, Papadimitrakopoulou V, Scardino PT, Gazdar AF, Wattenberg LW, Sporn MB, Sakr WA, Lippman SM, Von Hoff DD. Treatment and prevention of intraepithelial neoplasia: an important target for accelerated new agent development. Clin Cancer Res. 2002;8:314–46. - PubMed
-
- Dupont WD, Page DL. Risk factors for breast cancer in women with proliferative breast disease. N Engl J Med. 1985;312(3):146–51. - PubMed
-
- Arpino G, Laucirica R, Elledge RM. Premalignant and in situ breast disease: biology and clinical implications. Ann Intern Med. 2005;143:446–457. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

