FoxOs inhibit mTORC1 and activate Akt by inducing the expression of Sestrin3 and Rictor
- PMID: 20412774
- PMCID: PMC3031984
- DOI: 10.1016/j.devcel.2010.03.008
FoxOs inhibit mTORC1 and activate Akt by inducing the expression of Sestrin3 and Rictor
Abstract
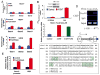
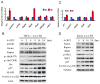
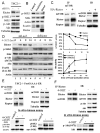
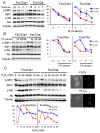
FoxO transcription factors and TORC1 are conserved downstream effectors of Akt. Here, we unraveled regulatory circuits underlying the interplay between Akt, FoxO, and mTOR. Activated FoxO1 inhibits mTORC1 by TSC2-dependent and TSC2-independent mechanisms. First, FoxO1 induces Sestrin3 (Sesn3) gene expression. Sesn3, in turn, inhibits mTORC1 activity in Tsc2-proficient cells. Second, FoxO1 elevates the expression of Rictor, leading to increased mTORC2 activity that consequently activates Akt. In Tsc2-deficient cells, the elevation of Rictor by FoxO increases mTORC2 assembly and activity at the expense of mTORC1, thereby activating Akt while inhibiting mTORC1. FoxO may act as a rheostat that maintains homeostatic balance between Akt and mTOR complexes' activities. In response to physiological stresses, FoxO maintains high Akt activity and low mTORC1 activity. Thus, under stress conditions, FoxO inhibits the anabolic activity of mTORC1, a major consumer of cellular energy, while activating Akt, which increases cellular energy metabolism, thereby maintaining cellular energy homeostasis.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures



References
-
- Bhaskar PT, Hay N. The Two TORCs and Akt. Dev Cell. 2007;12:487–502. - PubMed
-
- Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011–2015. - PubMed
-
- Du K, Herzig S, Kulkarni RN, Montminy M. TRB3: a tribbles homolog that inhibits Akt/PKB activation by insulin in liver. Science. 2003;300:1574–1577. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

