Thy-1 mRNA destabilization by norepinephrine a 3' UTR cAMP responsive decay element and involves RNA binding proteins
- PMID: 20412850
- PMCID: PMC2939224
- DOI: 10.1016/j.bbi.2010.04.006
Thy-1 mRNA destabilization by norepinephrine a 3' UTR cAMP responsive decay element and involves RNA binding proteins
Abstract
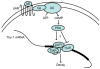
Thy-1 is a cell surface protein important in immunologic and neurologic processes, including T cell activation and proliferation, and neuronal outgrowth. In murine thymocytes, Thy-1 is downregulated in response to norepinephrine (NE) through posttranscriptional destabilization of its mRNA mediated by βAR/AC/cAMP/PKA signaling. In this study we investigated factors involved in NE/cAMP-mediated Thy-1 mRNA destabilization in S49 thymoma cells, and identified a region containing two copies of the AUUUA regulatory element (ARE), a motif commonly associated with mRNA decay, in the Thy-1 mRNA 3' UTR. Insertion of the Thy-1 ARE region into a reporter gene, resulted in cAMP induced destabilization of the reporter gene mRNA. RNA-protein binding studies revealed multiple Thy-1 ARE binding proteins, including AUF1, HuR, and TIAR. RNA silencing of HuR enhanced cAMP-mediated downregulation of Thy-1 mRNA, in contrast, silencing AUF1 had no effect. Immunoblotting revealed multiple proteins phosphorylated by PKA as a result of NE or cAMP signaling. These results reveal that the machinery of NE/cAMP modulation of Thy-1 mRNA decay involves a cAMP responsive ARE in its 3' UTR and multiple site specific ARE binding proteins. These findings add to our knowledge of Thy-1 mRNA regulation and provide insight into the regulation of ARE containing mRNAs, which impacts stress-related immunosuppression.
Published by Elsevier Inc.
Conflict of interest statement
Figures



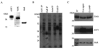
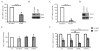

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

