Gamma-aminobutyric acid (GABA) is an autocrine excitatory transmitter in human pancreatic beta-cells
- PMID: 20413510
- PMCID: PMC2889769
- DOI: 10.2337/db09-0797
Gamma-aminobutyric acid (GABA) is an autocrine excitatory transmitter in human pancreatic beta-cells
Abstract
Objective: Paracrine signaling via gamma-aminobutyric acid (GABA) and GABA(A) receptors (GABA(A)Rs) has been documented in rodent islets. Here we have studied the importance of GABAergic signaling in human pancreatic islets.
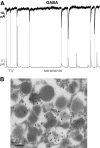
Research design and methods: Expression of GABA(A)Rs in islet cells was investigated by quantitative PCR, immunohistochemistry, and patch-clamp experiments. Hormone release was measured from intact islets. GABA release was monitored by whole-cell patch-clamp measurements after adenoviral expression of alpha(1)beta(1) GABA(A)R subunits. The subcellular localization of GABA was explored by electron microscopy. The effects of GABA on electrical activity were determined by perforated patch whole-cell recordings.
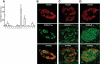
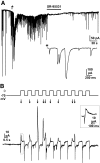
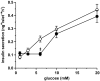
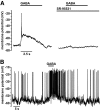
Results: PCR analysis detected relatively high levels of the mRNAs encoding GABA(A)R alpha(2), beta(3,) gamma(2), and pi subunits in human islets. Patch-clamp experiments revealed expression of GABA(A)R Cl(-) channels in 52% of beta-cells (current density 9 pA/pF), 91% of delta-cells (current density 148 pA/pF), and 6% of alpha-cells (current density 2 pA/pF). Expression of GABA(A)R subunits in islet cells was confirmed by immunohistochemistry. beta-Cells secreted GABA both by glucose-dependent exocytosis of insulin-containing granules and by a glucose-independent mechanism. The GABA(A)R antagonist SR95531 inhibited insulin secretion elicited by 6 mmol/l glucose. Application of GABA depolarized beta-cells and stimulated action potential firing in beta-cells exposed to glucose.
Conclusions: Signaling via GABA and GABA(A)R constitutes an autocrine positive feedback loop in human beta-cells. The presence of GABA(A)R in non-beta-cells suggests that GABA may also be involved in the regulation of somatostatin and glucagon secretion.
Figures
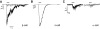

References
-
- Samols E, Bonner-Weir S, Weir GC: Intra-islet insulin-glucagon-somatostatin relationships. Clin Endocrinol Metab 1986;15:33–58 - PubMed
-
- Grapengiesser E, Salehi A, Qader SS, Hellman B: Glucose induces glucagon release pulses antisynchronous with insulin and sensitive to purinoceptor inhibition. Endocrinology 2006;147:3472–3477 - PubMed
-
- Hayashi M, Yamada H, Uehara S, Morimoto R, Muroyama A, Yatsushiro S, Takeda J, Yamamoto A, Moriyama Y: Secretory granule-mediated co-secretion of L-glutamate and glucagon triggers glutamatergic signal transmission in islets of Langerhans. J Biol Chem 2003;278:1966–1974 - PubMed
-
- Ishihara H, Maechler P, Gjinovci A, Herrera PL, Wollheim CB: Islet beta-cell secretion determines glucagon release from neighbouring alpha-cells. Nat Cell Biol 2003;5:330–335 - PubMed
-
- Rorsman P, Berggren PO, Bokvist K, Ericson H, Möhler H, Ostenson CG, Smith PA: Glucose-inhibition of glucagon secretion involves activation of GABAA-receptor chloride channels. Nature 1989;341:233–236 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

