Fingolimod (FTY720) enhances remyelination following demyelination of organotypic cerebellar slices
- PMID: 20413685
- PMCID: PMC2877831
- DOI: 10.2353/ajpath.2010.091234
Fingolimod (FTY720) enhances remyelination following demyelination of organotypic cerebellar slices
Abstract
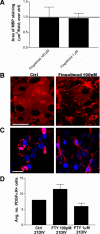
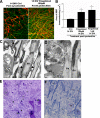
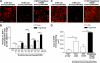
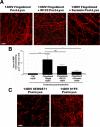
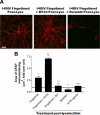
Remyelination, which occurs subsequent to demyelination, contributes to functional recovery and is mediated by oligodendrocyte progenitor cells (OPCs) that have differentiated into myelinating cells. Therapeutics that impact remyelination in the CNS could be critical determinants of long-term functional outcome in multiple sclerosis (MS). Fingolimod is a S1P receptor modulator in MS clinical trials due to systemic anti-inflammatory properties, yet may impact cells within the CNS by crossing the blood-brain barrier. Previous studies using isolated dissociated cultures indicate that neural cells express S1P receptors and respond to receptor engagement. Our objective was to assess the effects of fingolimod on myelin-related processes within a multicellular environment that maintains physiological cell-cell interactions, using organotypic cerebellar slice cultures. Fingolimod treatment had no impact on myelin under basal conditions. Fingolimod treatment subsequent to lysolecithin-induced demyelination enhanced remyelination and process extension by OPCs and mature oligodendrocytes, while increasing microglia numbers and immunoreactivity for the astrocytic marker glial fibrillary acidic protein. The number of phagocytosing microglia was not increased by fingolimod. Using S1P receptor specific agonists and antagonists, we determined that fingolimod-induced effects on remyelination and astrogliosis were mediated primarily through S1P3 and S1P5, whereas enhanced microgliosis was mediated through S1P1 and S1P5. Taken together, these data demonstrate that fingolimod modulates multiple neuroglial cell responses, resulting in enhanced remyelination in organotypic slice cultures that maintain the complex cellular interactions of the mammalian brain.
Figures


Comment in
-
The impact of fingolimod (FTY720) in neuroimmunologic diseases: mechanisms beyond immunomodulation.Am J Pathol. 2010 Jun;176(6):2599-601. doi: 10.2353/ajpath.2010.100200. Epub 2010 Apr 15. Am J Pathol. 2010. PMID: 20395429 Free PMC article.
References
-
- Griffiths I, Klugmann M, Anderson T, Yool D, Thomson C, Schwab MH, Schneider A, Zimmermann F, McCulloch M, Nadon N, Nave KA. Axonal swellings and degeneration in mice lacking the major proteolipid of myelin. Science. 1998;280:1610–1613. - PubMed
-
- Lappe-Siefke C, Goebbels S, Gravel M, Nicksch E, Lee J, Braun PE, Griffiths IR, Nave KA. Disruption of Cnp1 uncouples oligodendroglial functions in axonal support and myelination. Nat Genet. 2003;33:366–374. - PubMed
-
- LeBaron FN, Sanyal S, Jungalwala FB. Turnover rate of molecular species of sphingomyelin in rat brain. Neurochem Res. 1982;6:1081–1089. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

