Prolonged antigen survival and cytosolic export in cross-presenting human gammadelta T cells
- PMID: 20413723
- PMCID: PMC2889313
- DOI: 10.1073/pnas.1002769107
Prolonged antigen survival and cytosolic export in cross-presenting human gammadelta T cells
Abstract
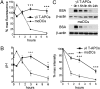
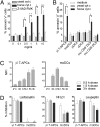
Human blood Vgamma9Vdelta2 T cells respond to signals from microbes and tumors and subsequently differentiate into professional antigen-presenting cells (gammadelta T-APCs) for induction of CD4(+) and CD8(+) T cell responses. gammadelta T-APCs readily take up and degrade exogenous soluble protein for peptide loading on MHC I, in a process termed antigen cross-presentation. The mechanisms underlying antigen cross-presentation are ill-defined, most notably in human dendritic cells (DCs), and no study has addressed this process in gammadelta T-APCs. Here we show that intracellular protein degradation and endosomal acidification were significantly delayed in gammadelta T-APCs compared with human monocyte-derived DCs (moDCs). Such conditions are known to favor antigen cross-presentation. In both gammadelta T-APCs and moDCs, internalized antigen was transported across insulin-regulated aminopeptidase (IRAP)-positive early and late endosomes; however, and in contrast to various human DC subsets, gammadelta T-APCs efficiently translocated soluble antigen into the cytosol for processing via the cytosolic proteasome-dependent cross-presentation pathway. Of note, gammadelta T-APCs cross-presented influenza antigen derived from virus-infected cells and from free virus particles. The robust cross-presentation capability appears to be a hallmark of gammadelta T-APCs and underscores their potential application in cellular immunotherapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Cresswell P, Ackerman AL, Giodini A, Peaper DR, Wearsch PA. Mechanisms of MHC class I–restricted antigen processing and cross-presentation. Immunol Rev. 2005;207:145–157. - PubMed
-
- Lin ML, Zhan Y, Villadangos JA, Lew AM. The cell biology of cross-presentation and the role of dendritic cell subsets. Immunol Cell Biol. 2008;86:353–362. - PubMed
-
- Villadangos JA, Schnorrer P. Intrinsic and cooperative antigen-presenting functions of dendritic-cell subsets in vivo. Nat Rev Immunol. 2007;7:543–555. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

