FcgammaRIIB in autoimmunity and infection: evolutionary and therapeutic implications
- PMID: 20414206
- PMCID: PMC4148599
- DOI: 10.1038/nri2762
FcgammaRIIB in autoimmunity and infection: evolutionary and therapeutic implications
Erratum in
- Nat Rev Immunol. 2010 Sep;10(9):674
Abstract
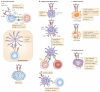
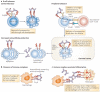
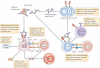
FcgammaRIIB is the only inhibitory Fc receptor. It controls many aspects of immune and inflammatory responses, and variation in the gene encoding this protein has long been associated with susceptibility to autoimmune disease, particularly systemic lupus erythematosus (SLE). FcgammaRIIB is also involved in the complex regulation of defence against infection. A loss-of-function polymorphism in FcgammaRIIB protects against severe malaria, the investigation of which is beginning to clarify the evolutionary pressures that drive ethnic variation in autoimmunity. Our increased understanding of the function of FcgammaRIIB also has potentially far-reaching therapeutic implications, being involved in the mechanism of action of intravenous immunoglobulin, controlling the efficacy of monoclonal antibody therapy and providing a direct therapeutic target.
Figures



References
-
- Nimmerjahn F, Ravetch JV. Fcγ receptors as regulators of immune responses. Nat. Rev. Immunol. 2008;8:34–47. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

