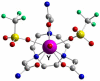
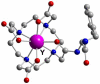
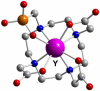
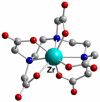
Coordinating radiometals of copper, gallium, indium, yttrium, and zirconium for PET and SPECT imaging of disease
- PMID: 20415480
- PMCID: PMC2874951
- DOI: 10.1021/cr900325h
Coordinating radiometals of copper, gallium, indium, yttrium, and zirconium for PET and SPECT imaging of disease
Figures

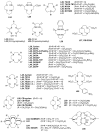
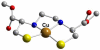
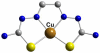











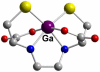



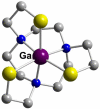
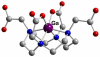
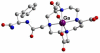
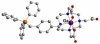

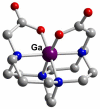
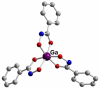
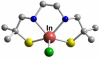
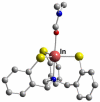


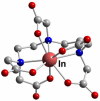

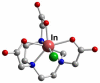
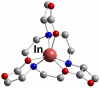
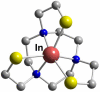

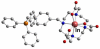
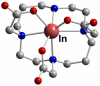

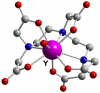



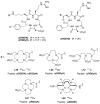


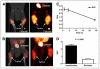
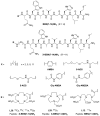
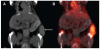
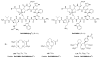
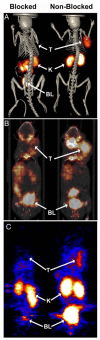
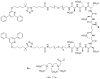
References
-
- Society of Nuclear Medicine 2009. http://interactive.snm.org/index.cfm?PageID=5571&RPID=969.
-
- Welch MJ, Redvanly CS, editors. Handbook of Radiopharmaceuticals: Radiochemistry and Applications. John Wiley & Sons Inc.; Hoboken, NJ: 2003.
-
- Blower PJ, Lewis JS, Zweit J. Nucl. Med. Biol. 1996;23(8):957. - PubMed
-
- Wadas TJ, Wong EH, Weisman GR, Anderson CJ. Curr. Pharm. Des. 2007;13(1):3. - PubMed
-
- Smith SV. IDrugs. 2005;8(10):827. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

