Candida albicans Hyr1p confers resistance to neutrophil killing and is a potential vaccine target
- PMID: 20415594
- PMCID: PMC3933264
- DOI: 10.1086/652407
Candida albicans Hyr1p confers resistance to neutrophil killing and is a potential vaccine target
Abstract
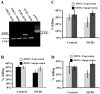
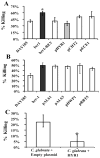
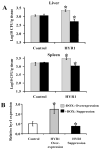
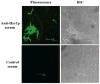
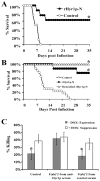
Candida albicans is the most common cause of invasive fungal infections in humans. It is unclear how C. albicans escapes from phagocytic attack and survives in the hostile blood environment during life-threatening systemic infections. Using a conditional overexpression or suppression genetic strategy, we discovered that HYR1 gene reduced phagocytic killing activity of C. albicans in vitro and increased tissue fungal burden in vivo. Concordant with its positive regulation by the transcription factor Bcr1p, autonomous expression of HYR1 complemented the hypersusceptibility to phagocyte-mediated killing of a bcr1 null mutant of C. albicans in vitro. As for C. albicans, heterologous expression of HYR1 in Candida glabrata rendered the organism more resistant to neutrophil killing activity. Vaccination with a recombinant Hyr1p significantly protected mice against hematogenously disseminated candidiasis (P = .001). Finally, anti-rHyr1p polyclonal antibodies enhanced mouse neutrophil killing activity by directly neutralizing rHyr1p effects in vitro. Thus, Hyr1 is an important virulence factor for C. albicans, mediating resistance to phagocyte killing. Hyr1p is a promising target for vaccine or other immunological or small molecule intervention to improve the outcomes of disseminated candidiasis.
Figures

Comment in
-
Hyr1 protein and β-glucan conjugates as anti-Candida vaccines.J Infect Dis. 2010 Dec 15;202(12):1930. doi: 10.1086/657417. J Infect Dis. 2010. PMID: 21083372 No abstract available.
References
-
- Spellberg BJ, Filler SG, Edwards JE., Jr Current treatment strategies for disseminated candidiasis. Clin Infect Dis. 2006;42:244–251. - PubMed
-
- Gulay Z, Imir T. Anti-candidial activity of natural killer (NK) and lymphokine activated killer (LAK) lymphocytes in vitro. Immunobiology. 1996;195:220–230. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

