The immunology of tuberculosis: from bench to bedside
- PMID: 20415982
- PMCID: PMC5463744
- DOI: 10.1111/j.1440-1843.2010.01739.x
The immunology of tuberculosis: from bench to bedside
Abstract
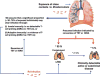
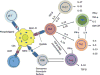
Tuberculosis (TB) is an international public health priority and kills almost two million people annually. TB is out of control in Africa due to increasing poverty and HIV coinfection, and drug-resistant TB threatens to destabilize TB control efforts in several regions of the world. Existing diagnostic tools and therapeutic interventions for TB are suboptimal. Thus, new vaccines, immunotherapeutic interventions and diagnostic tools are urgently required to facilitate TB control efforts. An improved understanding of the immunopathogenesis of TB can facilitate the identification of correlates of immune protection, the design of effective vaccines, the rational selection of immunotherapeutic agents, the evaluation of new drug candidates, and drive the development of new immunodiagnostic tools. Here we review the immunology of TB with a focus on aspects that are clinically and therapeutically relevant. An immunologically orientated approach to tackling TB can only succeed with concurrent efforts to alleviate poverty and reduce the global burden of HIV.
Figures


References
-
- World Health Organization. Global Tuberculosis Control 2009: Epidemiology, Strategy, Financing. WHO; Geneva: 2009.
-
- World Health Organization. Anti-Tuberculosis Drug Resistance in the World: The WHO/IUATLD Global Project on Anti-Tuberculosis Drug Resistance Surveillance. WHO; Geneva: 2008.
-
- Hanekom WA, Abel B, Scriba TJ. Immunological protection against tuberculosis. S Afr Med J. 2007;97:973–7. - PubMed
-
- Reece ST, Kaufmann SH. Rational design of vaccines against tuberculosis directed by basic immunology. Int J Med Microbiol. 2008;298:143–50. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

