Structural characterization of acylimine-containing blue and red chromophores in mTagBFP and TagRFP fluorescent proteins
- PMID: 20416505
- PMCID: PMC2862997
- DOI: 10.1016/j.chembiol.2010.03.005
Structural characterization of acylimine-containing blue and red chromophores in mTagBFP and TagRFP fluorescent proteins
Abstract
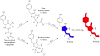
We determined the 2.2 A crystal structures of the red fluorescent protein TagRFP and its derivative, the blue fluorescent protein mTagBFP. The crystallographic analysis is consistent with a model in which TagRFP has the trans coplanar anionic chromophore with the conjugated pi-electron system, similar to that of DsRed-like chromophores. Refined conformation of mTagBFP suggests the presence of an N-acylimine functionality in its chromophore and single C(alpha)-C(beta) bond in the Tyr64 side chain. Mass spectrum of mTagBFP chromophore-bearing peptide indicates a loss of 20 Da upon maturation, whereas tandem mass spectrometry reveals that the C(alpha)-N bond in Leu63 is oxidized. These data indicate that mTagBFP has a new type of the chromophore, N-[(5-hydroxy-1H-imidazole-2-yl)methylidene]acetamide. We propose a chemical mechanism in which the DsRed-like chromophore is formed via the mTagBFP-like blue intermediate.
(c) 2010 Elsevier Ltd. All rights reserved.
Figures
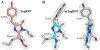

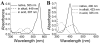

References
-
- Barondeau DP, Kassmann CJ, Tainer JA, Getzoff ED. The case of the missing ring: radical cleavage of a carbon-carbon bond and implications for GFP chromophore biosynthesis. J Am Chem Soc. 2007;129:3118–3126. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

