Methotrexate-induced side effects are not due to differences in pharmacokinetics in children with Down syndrome and acute lymphoblastic leukemia
- PMID: 20418240
- PMCID: PMC2895034
- DOI: 10.3324/haematol.2009.019778
Methotrexate-induced side effects are not due to differences in pharmacokinetics in children with Down syndrome and acute lymphoblastic leukemia
Abstract
Background: Children with Down syndrome have an increased risk of developing acute lymphoblastic leukemia and a poor tolerance of methotrexate. This latter problem is assumed to be caused by a higher cellular sensitivity of tissues in children with Down syndrome. However, whether differences in pharmacokinetics play a role is unknown.
Design and methods: We compared methotrexate-induced toxicity and pharmacokinetics in a retrospective case-control study between patients with acute lymphoblastic leukemia who did or did not have Down syndrome. Population pharmacokinetic models were fitted to data from all individuals simultaneously, using non-linear mixed effect modeling.
Results: Overall, 468 courses of methotrexate (1-5 g/m(2)) were given to 44 acute lymphoblastic leukemia patients with Down syndrome and to 87 acute lymphoblastic leukemia patients without Down syndrome. Grade 3-4 gastrointestinal toxicity was significantly more frequent in the children with Down syndrome than in those without (25.5% versus 3.9%; P=0.001). The occurrence of grade 3-4 gastrointestinal toxicity was not related to plasma methotrexate area under the curve. Methotrexate clearance was 5% lower in the acute lymphoblastic leukemia patients with Down syndrome (P=0.001); however, this small difference is probably clinically not relevant, because no significant differences in methotrexate plasma levels were detected at 24 and 48 hours.
Conclusions: We did not find evidence of differences in the pharmacokinetics of methotrexate between patients with and without Down syndrome which could explain the higher frequency of gastrointestinal toxicity and the greater need for methotrexate dose reductions in patients with Down syndrome. Hence, these problems are most likely explained by differential pharmaco-dynamic effects in the tissues between children with and without Down syndrome. Although the number of patients was limited to draw conclusions, we feel that it may be safe in children with Down syndrome to start with intermediate dosages of methotrexate (1-3 g/m(2)) and monitor the patients carefully.
Figures

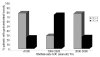
References
-
- Weijerman ME, van Furth AM, Vonk Noordegraaf A, van Wouwe JP, Broers CJ, Gemke RJ. Prevalence, neonatal characteristics, and first-year mortality of Down syndrome: a national study. J Pediatr. 2008;152(1):15–9. - PubMed
-
- Whitlock JA, Sather HN, Gaynon P, Robison LL, Wells RJ, Trigg M, et al. Clinical characteristics and outcome of children with Down syndrome and acute lymphoblastic leukemia: a Children’s Cancer Group study. Blood. 2005;106(13):4043–9. - PubMed
-
- Dordelmann M, Schrappe M, Reiter A, Zimmermann M, Graf N, Schott G, et al. Down’s syndrome in childhood acute lymphoblastic leukemia: clinical characteristics and treatment outcome in four consecutive BFM trials. Berlin-Frankfurt-Munster Group. Leukemia. 1998;12(5):645–51. - PubMed
-
- Zeller B, Gustafsson G, Forestier E, Abrahamsson J, Clausen N, Heldrup J, et al. Nordic Society of Paediatric Haematology and and Oncology (NOPHO) Acute leukaemia in children with Down syndrome: a population-based Nordic study. Br J Haematol. 2005;128(6):797–804. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

