Structure, function, and insights into the biosynthesis of a head-to-head hydrocarbon in Shewanella oneidensis strain MR-1
- PMID: 20418444
- PMCID: PMC2893466
- DOI: 10.1128/AEM.00433-10
Structure, function, and insights into the biosynthesis of a head-to-head hydrocarbon in Shewanella oneidensis strain MR-1
Abstract
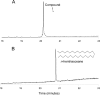
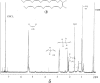
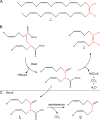
A polyolefinic hydrocarbon was found in nonpolar extracts of Shewanella oneidensis MR-1 and identified as 3,6,9,12,15,19,22,25,28-hentriacontanonaene (compound I) by mass spectrometry, chemical modification, and nuclear magnetic resonance spectroscopy. Compound I was shown to be the product of a head-to-head fatty acid condensation biosynthetic pathway dependent on genes denoted as ole (for olefin biosynthesis). Four ole genes were present in S. oneidensis MR-1. Deletion of the entire oleABCD gene cluster led to the complete absence of nonpolar extractable products. Deletion of the oleC gene alone generated a strain that lacked compound I but produced a structurally analogous ketone. Complementation of the oleC gene eliminated formation of the ketone and restored the biosynthesis of compound I. A recombinant S. oneidensis strain containing oleA from Stenotrophomonas maltophilia strain R551-3 produced at least 17 related long-chain compounds in addition to compound I, 13 of which were identified as ketones. A potential role for OleA in head-to-head condensation was proposed. It was further proposed that long-chain polyunsaturated compounds aid in adapting cells to a rapid drop in temperature, based on three observations. In S. oneidensis wild-type cells, the cellular concentration of polyunsaturated compounds increased significantly with decreasing growth temperature. Second, the oleABCD deletion strain showed a significantly longer lag phase than the wild-type strain when shifted to a lower temperature. Lastly, compound I has been identified in a significant number of bacteria isolated from cold environments.
Figures

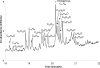

References
-
- Albro, P. W., and J. C. Dittmer. 1969. The biochemistry of long-chain nonisoprenoid hydrocarbons. I. Characterization of the hydrocarbons of Sarcina lutea and the isolation of possible intermediates of biosynthesis. Biochemistry 8:394-405. - PubMed
-
- Albro, P. W., and J. C. Dittmer. 1969. The biochemistry of long-chain, nonisoprenoid hydrocarbons. II. The incorporation of acetate and the aliphatic chain is isoleucine and valine into fatty acids and hydrocarbons by Sarcina lutea in vivo. Biochemistry 8:953-959. - PubMed
-
- Albro, P. W., and J. C. Dittmer. 1969. The biochemistry of long-chain nonisoprenoid hydrocarbons. III. The metabolic relationship of long-chain fatty acids and hydrocarbons and other aspects of hydrocarbon metabolism in Sarcina lutea. Biochemistry 8:1913-1918. - PubMed
-
- Albro, P. W., and J. C. Dittmer. 1969. The biochemistry of long-chain nonisoprenoid hydrocarbons. IV. Characteristics of synthesis by a cell-free preparation of Sarcina lutea. Biochemistry 8:3317-3324. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

