Alterations in membrane type-1 matrix metalloproteinase abundance after the induction of thoracic aortic aneurysm in a murine model
- PMID: 20418476
- PMCID: PMC2904124
- DOI: 10.1152/ajpheart.00028.2010
Alterations in membrane type-1 matrix metalloproteinase abundance after the induction of thoracic aortic aneurysm in a murine model
Abstract
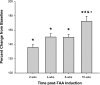
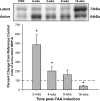
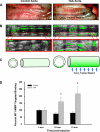
Thoracic aortic aneurysms (TAAs) develop as a result of dysregulated extracellular matrix remodeling mediated by several matrix metalloproteinases (MMPs). Membrane type-1 MMP (MT1-MMP) is the prototypical member of a unique family of membrane-bound MMPs, possessing multiple substrates and functions. The present study tested the hypothesis that MT1-MMP expression, abundance, and activity would be elevated during TAA development and that this protease is produced primarily by mesenchymal cells within the thoracic aorta. Descending thoracic aortas were harvested from C57BL/6J mice at multiple time points (2, 4, 8, and 16 wk, n = 15 each) post-TAA induction (0.5M CaCl(2), 15 min) and compared with reference controls (n = 15). The expression and abundance of MT1-MMP, MMP-2, and tissue inhibitor of metalloproteinase (TIMP)-2 were assessed by quantitative PCR and immunoblot analysis. MT1-MMP activity was determined by fluorescent peptide assay. MT1-MMP was localized within the aortic wall by immunohistochemistry. MT1-MMP abundance and localization in live animals (8 wk post-TAA induction vs. control) was determined by micro-ultrasound imaging with an MT1-MMP-targeted microbubble contrast agent. Aortic diameter was increased 172 +/- 7% at 16 wk post-TAA induction (P < 0.05). MT1-MMP and MMP-2 mRNA levels were elevated at 2 wk post-TAA induction (P < 0.05). MT1-MMP protein abundance increased progressively to a maximum of 178 +/- 26% at 16 wk post-TAA induction, whereas MMP-2 and TIMP-2 peaked at 2 wk post-TAA induction (526 +/- 93% and 376 +/- 48%, respectively, P < 0.05). MT1-MMP colocalized with fibroblasts, and MT1-MMP-targeted contrast binding was elevated in 8-wk TAA-induced mice versus control mice (217 +/- 53% vs. 81 +/- 8%, P < 0.05). In conclusion, these novel results suggest that MT1-MMP plays a dynamic multifunctional role in TAA development and, therefore, may provide a significant target for therapeutic strategies.
Figures



References
-
- Annabi B, Shedid D, Ghosn P, Kenigsberg RL, Desrosiers RR, Bojanowski MW, Beaulieu E, Nassif E, Moumdjian R, Beliveau R. Differential regulation of matrix metalloproteinase activities in abdominal aortic aneurysms. J Vasc Surg 35: 539–546, 2002 - PubMed
-
- Barbour JR, Spinale FG, Ikonomidis JS. Proteinase systems and thoracic aortic aneurysm progression. J Surg Res 139: 292–307, 2007 - PubMed
-
- Bickerstaff LK, Pairolero PC, Hollier LH, Melton LJ, Van Peenen HJ, Cherry KJ, Joyce JW, Lie JT. Thoracic aortic aneurysms: a population-based study. Surgery 92: 1103–1108, 1982 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

